June 12, 2018 report
Improving electron transfer in enzymatic biofuel cells
A team of researchers with members from institutions in Singapore, China and the U.K. has found a way to improve electron transfer in enzymatic biofuel cells. In their paper published in the journal Nature Energy, they describe their technique and how well it works. Huajie Yin and Zhiyong Tang with Griffith University in Australia and the National Center for Nanoscience and Technology in China, offer a News & Views piece on the work done by the team in the same journal issue.
Enzymatic biofuel cells are, as their name implies, a type of fuel cell based on enzymes as catalysts instead of expensive metals. Because of their potential, scientists have been eager to find ways to overcome problems that have inhibited commercial applications—they are expected to be much cheaper to make than those now in use.
Currently, enzymatic biofuel cells are inefficient, have a short lifespan and do not produce much power. These problems, the researchers note, are due to the difficulty in wiring enzymes and electrode surfaces. In this effort, they claim to have overcome some of that difficulty by combining two previously developed methods aimed at solving the problem. The first method involves connecting an enzyme to the surface of an electrode in such a way as to allow the electrons to tunnel between the two—it is called direct electron transfer. The second method involves a mediator that is used help the transfer—it is called, quite naturally, mediated electron transfer.
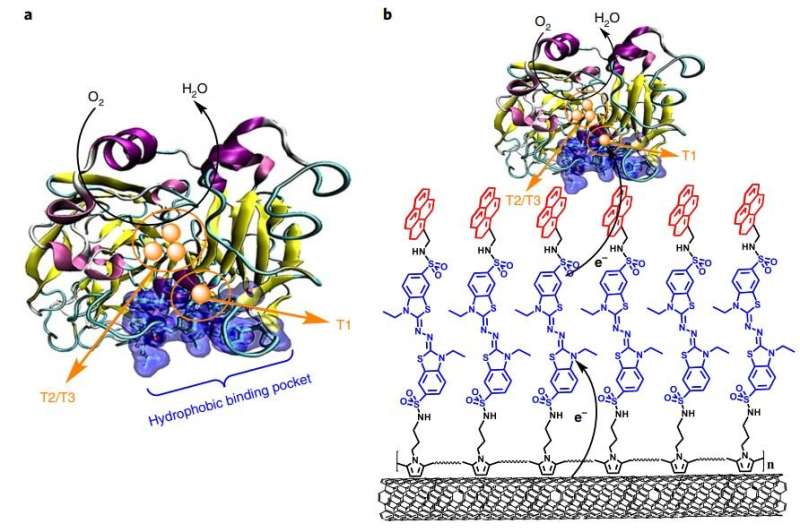
The researchers combined the two approaches to take advantage of the benefits of each. They used laccase as the enzyme and designed a transfer system that connected to a special type of carbon nanotube surface to further improve electron transfer. The system was made of three parts, an ABTS compound (to serve as a mediator), situated between a polypyrrole group at one end and a pyrene group at the other.
In testing their technique, the team found that the maximum OOR current density reached as high as 2.45 mA/cm2 and their device was able to keep half of its ORR current for 120 days. They suggest their results show promise and expect further improvements as they refine the technique.
More information: Kamal Elouarzaki et al. Coupling orientation and mediation strategies for efficient electron transfer in hybrid biofuel cells, Nature Energy (2018). DOI: 10.1038/s41560-018-0166-4
Abstract
Enzymes are promising electrocatalysts for electron transfer (ET) in many biological processes. Strategies to enhance ET between enzymes and electroactive surfaces include orientation and immobilization of the enzymes and electron mediation. Here, we develop a strategy to couple orientation and electron mediation on electrodes based on carbon nanotubes. This is achieved by the synthesis of a redox mediator that contains an enzyme-orientation site (pyrene), an electron-carrier redox mediator (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS)) and an electropolymerizable monomer (pyrrole). The coupling of an enzymatic orientation and a mediated ET in the same chemical structure (pyrrole–ABTS–pyrene (pyrr–ABTS–pyr)) provides a much-improved performance in the bioelectrocatalysis. We demonstrate two fuel cells for the synthesized redox mediator. In a proton-exchange membrane hydrogen/air fuel cell and in a membraneless fuel cell, the pyrr–ABTS–pyr biocathode provides a power density of 1.07 mW cm−2 and 7.9 mW cm−2, respectively. The principle of coupling an enzyme orientation and a redox mediator allows a great variety of mediators to be engineered and provides vast possibilities for the development of fuel cells.
Journal information: Nature Energy
© 2018 Tech Xplore