Research opens doors to expanded DNA studies
Dr. Wonmuk Hwang, associate professor in the Department of Biomedical Engineering at Texas A&M University, is researching the mechanics of DNA, the blueprint of the human body.
Hwang and his former doctoral student, Dr. Xiaojing Teng, zoomed into the question: if the genetic information is the same in all cells, as it should be, why do muscle cells look and act differently than skin cells?
"To selectively turn on and off different genes to determine the cell type, you need to modify this gene expression, and one way to do that is to chemically modify DNA," Hwang said.
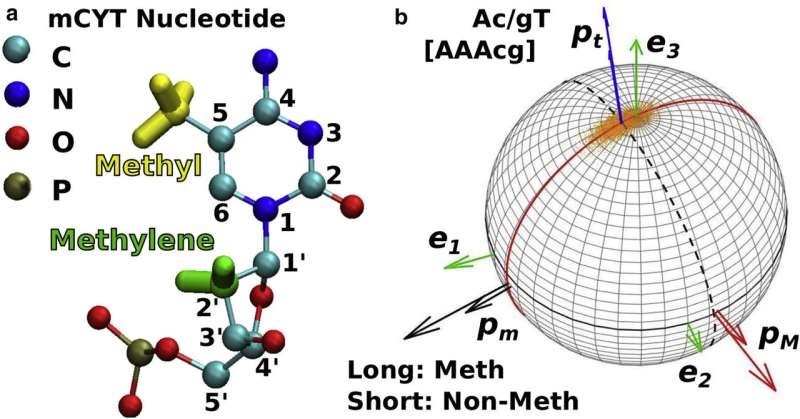
A major way the body achieves this is through methylation, where methyl groups stick to a particular location in DNA, so that the group blocks the genetic information in this region from being read by the cell. In addition, methylation affects local flexibility of DNA, which in turn controls how DNAs are packaged into chromosomes. While these processes were generally known, how methylation affects DNA's mechanical properties has remained unknown.
Through extensive simulations using supercomputers at the Texas A&M High Performance Research Computing Facility, as well as the Texas Advanced Computing Center at The University of Texas at Austin, Hwang was able to determine how the areas of DNA around the methyl groups bump against each other and alter mechanical behaviors.
Along with that discovery, Hwang said they found another unexpected insight.
"These methyl groups not only bump against neighboring atoms in DNA, but water molecules rearrange around these methyl groups," Hwang said. "The rearranged water molecules actually resist deformation even in the absence of the direct collision of atoms with the methyl groups, as if the surrounding water molecules are a part of DNA itself."
There are multiple applications to understanding how processes such as methylation work. One example Hwang gave was developing more knowledge on how cancer cells function.
"Cancer cells often methylate their DNA to turn off genes that control cell division, promoting uncontrolled growth," he said.
Another is drug interaction with DNA and how drug design should take these water molecules into account.
"This study won't immediately lead to a new drug, but it provides one more step toward more rational drug design," Hwang said. "People have been working on cancer for decades, and I don't claim that I can solve the problem right away. But all of these efforts make step-by-step progress in the right direction."
Hwang said the method developed by his team opens the door to analyzing other types of DNA or RNA modifications and how their behavior changes depending on what drugs are introduced.
The article was featured on the cover of the April 24 issue of Biophysical Journal.
More information: Xiaojing Teng et al, Effect of Methylation on Local Mechanics and Hydration Structure of DNA, Biophysical Journal (2018). DOI: 10.1016/j.bpj.2018.03.022
Journal information: Biophysical Journal
Provided by Texas A&M University