May 18, 2018 report
Wisdom of the protists; electron flow tricks for controlling cancer
All schoolchildren learn that the difference between eukaryotes and prokaryotes has something to do with a nucleus. This is usually around the same time they learn that the mitochondria is the powerhouse of the cell. The real difference between these two life forms, however, has more to do with how they control the flow of electrons to make their living, i.e., their electron transport chains going from donors to acceptors via redox reactions.
We can state this distinction more directly. eukaryotes run one very specialized version of the electron transport chain while procaryotes run a more generalized system of multiple simultaneously operating electron transport chains. In eukaryotes, it looks something like this:
NADH → Complex I → Q → Complex III → cytochrome c → Complex IV → O2
NADH (dehydrogenase) is the electron donor, Complexes I, III and IV are proton pumps, Q is the membrane soluble mobile electron carriers—the quinone pool, and cytochrome c the soluble electron carrier
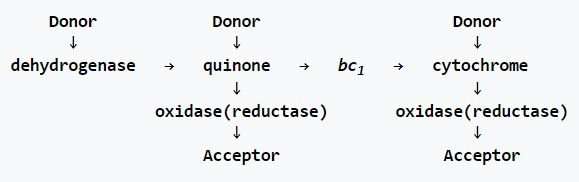
The prokaryotic chain (bacteria and archaea), on the other hand, includes multiple donors that can input electrons at three levels. Not only are there typically several different initial dehydrogenases used here, there are often different membrane soluble electron carriers that can contribute to the quinone pool, and multiple oxidases and reductases. Borrowing directly from Wikipedia, the generalized schematic looks like this:
Although eukaryotes use just one instance of this chain, they have been able to borrow a few tricks from prokaryotes that they can call upon when needed. Under certain circumstances, reverse electron flow can occur at one or more of the respiratory complexes. One thing we left out in the eukaryotic chain above is complex II, which is a branch point that feeds into the Q pool. In a post here on Wednesday, we discussed in some detail how this particular enzyme system (Succinate dehydrogenase) is regulated in the brain by GABA to regulate mitochondrial access to purine nucleotides. Succinate dehydrogenase is also a major stop on the citric acid cycle. When there are local reversals in the direction of the citric acid cycle here, there must also be reverse electron transport at complex II in respiration.
Quinones I have known and loved
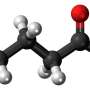
A recent paper published in eLife describes how and when this occurs in a peculiar protist called pygsuia. The authors discovered that this organism makes a special kind of quinone using an enzyme that it acquired from bacteria by horizontal gene transfer. Pysugia is an anaerobe and it no longer retains full blown mitochondria. Instead, it uses remnant organelles similar to hydrogenosomes. Instead of using coenzyme Q10 (ubiquinone), like humans use in their quinone pool, Pysugia modifies ubiquinone to a molecule called rhodoquinone (RQ) using a specific methyltransferase to replace one methoxy group with an amine.
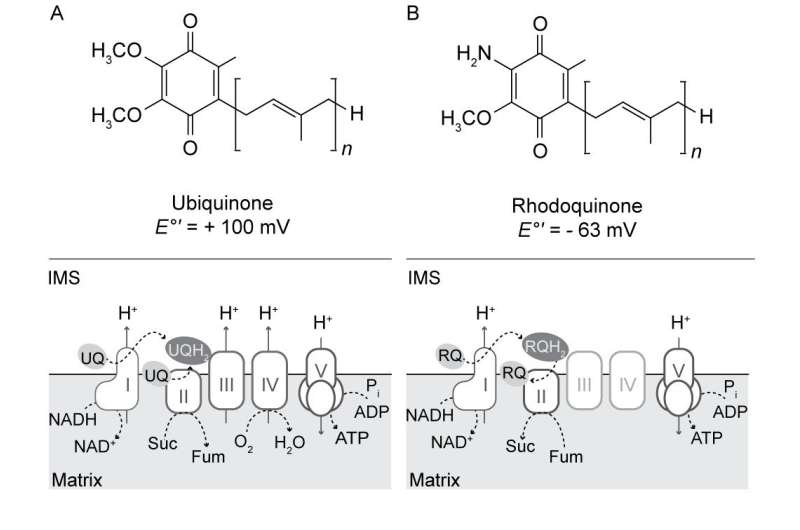
The beauty of RQ is that it retains all the structural assets that make ubiquinone so useful—essentials like multiple oxidation states and adjustable length lipid soluble isoprenoid tails for membranes of different thicknesses—but has a reduction potential significantly more negative than ubiquinone (-63 mv vs +100mv). What this means for electron transport is that the reverse reaction at complex II, namely the reduction of fumarate to succinate, becomes much more favorable. When there is no oxygen around (which is normally the terminal electron acceptor used at complex IV), there is little reason for organisms like pysugia to retain any respiratory complexes beyond succinate dehydrogenase. Therefore, they reoxidize RQH2 back to its original RQ form at complex II and generate succinate. Complex I can then cycle again.
RQ is not the only alternative to ubiquinone. Bacteria frequently make use of the menaquinone (aka vitamin K2), which is the molecule we utilize for carboxylation of glutamate residues in our coagulation pathway proteins. Plants use phylloquinone (vitamin K1) in photosystem I, and plastoquinone in photosystem II, which has methyl groups in place of ubiquinol's methoxy groups. Some makers of dubious anti-aging miracle molecules have been known to peddle various plastoquinone-related derivatives. One potential supplement called SkQ1 was specifically designed to penetrate mitochondrial membranes. Another, SkQR1 is a rhodamine-containing analog that has antioxidant and protonophore activity.
Many mammalian parasites have a life cycle that requires transit through tissues with widely different oxygen levels in order for them to mature. Some of them, like the ascaris nematode, have managed to get the best of both worlds. They can print off different versions of complex II subunit, along with different Q pool electron carriers, and optimally run reverse electron transport as oxygen levels require. Adult ascaris worms live in low-oxygen intestines and expel their eggs with the feces of their host. When a larva is ingested by a new host, it hatches and invades its intestinal wall, then proceeds to migrate through the host's organs until it eventually winds up in the lungs, where oxygen alters development.
By some hideous magic only a parasite could appreciate, ascaris causes the host to violently cough up the maturing larva, after which they are subsequently swallowed and directed again to the small intestine. Here, they turn off production of complex III and IV, but can continue to make ATP by pumping protons and oxidizing NADH at complex I while recycling RQ at complex II. When organisms like ascaris, or E. coli, or C. elegans maintain dedicated enzymes for running reverse electron transport at complex II, these enzymes are called fumarate reductases as opposed to succinate dehydrogenases.
If you need to kill any such parasite lurking within you, this can be a special thing. Researchers have uncovered compounds, like nafuredin, that specifically inhibit the NADH dehydrogenase complex in the mitochondria of helminths. Similarly, atpenin and flutolanil inhibit complex II at its quinone binding site. To appreciate why we said 'controlling cancer' in the headline, we need to open one final gift from science:
Many human cancers can thrive in poorly vascularized centers of tumors or other low-oxygen pockets in the body. Of particular note, fumarate respiration has been observed in many kinds of cancer.
Ascaris makes different varieties of each of Complex II's four subunits; a flavoprotein subunit (Fp), iron–sulfur subunit (Ip), cytochrome b large subunit (CybL), and cytochrome b small subunit (CybS). Humans don't have special RQ quinones, nor do they keep any bonafide fumarate reductase enzymes on tap. However, they do have two separate versions of the Fp subunit, and produce them both in most tissues. Researchers have found that many cancers including breast, lung and lymphoma preferentially make the type II Fp subunit.
Genetic sequencing has revealed that several varieties of tumors, like pheochromocytoma and paraganglioma, are associated wth certain variants of complex II genes. These tumors are also associated with specific assembly factors that help construct and position complex II. Complex II is unique in that all its subunits are encoded in the nucleus. There is still some debate about where and how many complex II units get coordinated into larger respiratory supercomplexesthat can confine the Q and cytochrome C mobile electron carriers. It is now understood that the translation and early assembly of complex III and complex IV occur at the inner membrane boundary while that of complex V takes place deep in the cristae.
Today, many new therapies depend on which particular variants you and your tumor happen to have. Before antihelminthic compounds become a standard of care tumor treatments, there are several other important enzyme systems that deal in quinones that may need to be fully investigated. For example, these include quinone biosynthesis enzymes, alternative oxidase, glycerol-3-phosphate dehydrogenase, and dihydroorotate dehydrogenase (DHODH).
DHODH is a critical step in de novo pyrimidine biosynthesis and requires complex III to regenerate ubiquinone in order to function. Appreciating these many subtle links between electron transport and synthesis of the fundamental building blocks of life is critical to understanding what cancer is.
More information: Courtney W Stairs et al. Microbial eukaryotes have adapted to hypoxia by horizontal acquisitions of a gene involved in rhodoquinone biosynthesis, eLife (2018). DOI: 10.7554/eLife.34292
Journal information: eLife
© 2018 Phys.org