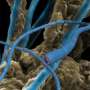
A single cable bacteria filament (about 1 cm long) stretches from sediment (far left) to oxygen (far right). Credit: Jesper Tataru Bjerg, Aarhus University
An international research group has shed new light on cable bacteria. Using laser light, researchers have followed electrons as they travel through the current-conducting bacteria, and on the basis of the electrical potential in the bacteria, they have calculated that the bacteria cannot function efficiently at depths exceeding 3 cm into the sediment due to voltage loss.
Together with colleagues from the Netherlands and Austria, researchers from Aarhus University have used laser spectrometry as an advanced voltmeter to follow electrons through cable bacteria over millimetre-long distances, which are a thousand times longer than previously measured in any living organism.
Using their measurements, the researchers also calculated the voltage loss through an individual cable bacterium (approx. twelve to 14 millivolts per mm), and thus also calculated how far down into the oxygen-free seabed they can reach without losing their ability to conduct electricity: "They'll be in trouble if they stretch further than three centimeters downward into the sediment. In principle, the individual bacteria can be longer than three centimeters, but then they must meander up and down, so that they alternate between the oxygen-rich and oxygen-free environments in the sediment," explains Professor Andreas Schramm from the Center for Electromicrobiology (CEM) at Aarhus University.
A muddy picture
The CEM basic research centre was set up in 2017 to find answers to some of the questions that mushroomed after the discovery of these living electrical cables in the seabed under the Aarhus Bugt seven years ago.
How can a living biological structure act as an efficient electrical conductor? How does the cable bacterium distribute energy between cells? And how do they use the energy? At that time, researchers had only a muddy picture of what was going on in these long, thin bacteria. The bacteria transport electrons from the oxygen-free mud a couple of centimetres down in the seabed to the oxygen-rich mud and silt on the surface, making it possible for them to eat with one end and breathe with the other.
Hundreds of cable bacteria emerge out of the sediment at the bottom of the video and crawl outwards, hoping to find oxygen. We use their willingness to search for oxygen when we want to do experiments with single cable bacteria. Video: AU
After observing live cable bacteria under the microscope and exposing them to resonance Raman spectroscopy, the research group has come closer to one of the answers. Their results are published in the scientific journal PNAS on May 7th.
Raman spectroscopy illuminates molecules with laser light. The frequency distribution of the scattered light makes it possible to read the energy level of the molecules.
"In this context, we have used the instrument as an advanced voltmeter, that we have targeted towards a specific type of proteins, cytochromes, in the cables," said first author of the publication, Jesper T. Bjerg, PhD student at Aarhus University.
Power cut
Head of CEM, Professor Lars Peter Nielsen, says, "All living cells move electrons around and try to park them in so-called cytochromes. The more free parking spaces there are, the higher the electrical potential. With our advanced voltmeter, we have now measured the available parking spaces and thus the electrical potential of each cytochrome along the wires of individual cable bacteria, while these wires conduct electrons from one end of the bacteria to the other. Our measurements showed the lowest potential in the cells at the end where electrons from the food source were being loaded, and the highest potential at the opposite end, where the electrons were being unloaded to oxygen."
As part of the study, the researchers cut off the upper end of the bacteria (i.e. the end that transfers electrons to the oxygen in the water) with a laser. This led to a rapid decline in the electrical potential in the remaining part of the bacteria, indicating that the parking spaces in the cytochromes were filled with electrons that could not get any further because of the electricity cut.
"This is the first time that electron transport has been demonstrated in individual cable bacteria. At the same time, we have used a well-established method, proving the results from our initial measurements with unconventional methods in opaque mud columns," said Lars Peter Nielsen.
More information: Jesper T. Bjerg el al., "Long-distance electron transport in individual, living cable bacteria," PNAS (2018). www.pnas.org/cgi/doi/10.1073/pnas.1800367115
Journal information: Proceedings of the National Academy of Sciences
Provided by Aarhus University