Improved understanding of groundbreaking liquid-metal 2-D technique
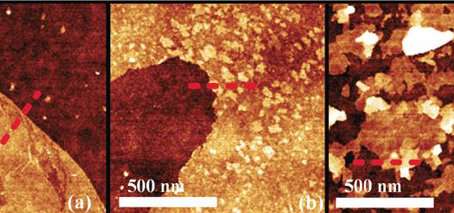
Last year, FLEET researchers at RMIT developed a ground-breaking new method of depositing atomically-thin (two-dimensional) crystals using molten metals, described as a 'once-in-a-decade' advance.
Earlier this year, the same research team expanded the new method from controlled to ambient conditions, and has properly characterised the growth mechanisms for key tin oxides, which should allow improved control of target oxide growth.
The technique developed at RMIT last year introduced liquid metals (gallium-based) as a successful reaction environment for the synthesis of desirable, atomically-thin oxides that were unattainable using prior methods. It's a process so cheap and simple that it could be done on a kitchen stove by a non-scientist.
While the initial study used costly, specially-designed alloys and an often tightly-controlled environment, this latest research has confirmed that high-quality 2-D materials can be formed in ambient conditions using cheaper liquid tin, simplifying future research and applications.
Researchers also characterised the growth mechanism for the first time, creating a 'road map' of crystal formation and growth. Such growth proved surprisingly complex, with small 'islands' of tin-oxides (SnOx) forming on larger, perfect 2-D tin monoxide (SnO) monolayers, before thickening and taking on more oxygen to become tin dioxide (SnO2).
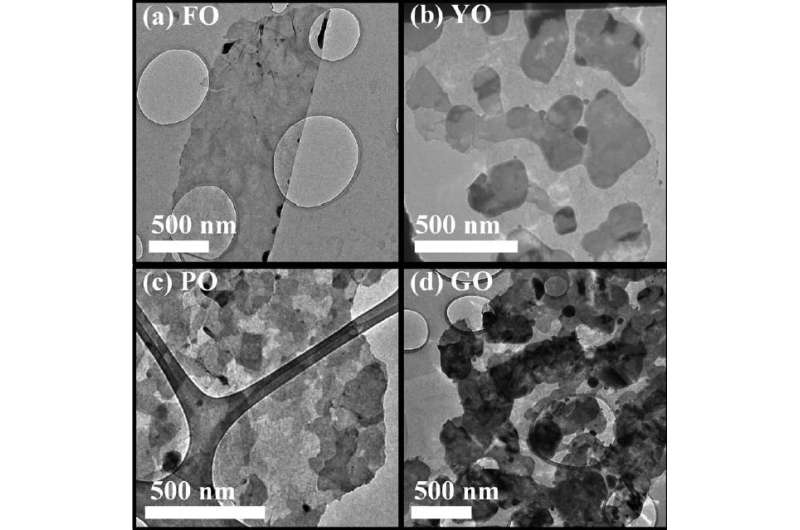
Future applications
This simple, repeatable method of growing 2-D tin-oxide crystals can be expanded to other low-melting point liquid metals and their alloys.
Having properly characterised growth mechanisms, researchers believe it should be possible to control the rate of surface oxide formation by careful control of the atmospheric oxygen content, and hence control the number and thickness of oxide layers and resulting stoichiometry.
Tin oxides are of particular interest as 2-D materials. Electronically, they can be both p-type (SnO) or n-type (SnO2) semiconductors, which is of great interest to field effect transistor (FET) designers.
Evolution of 2-D tin oxides on the surface of molten tin was published in Chemical Communications in January 2018.
The study was conducted using the facilities and expertise of the Australian Microscopy and Microanalysis Research Facility at the RMIT Microscopy & Microanalysis Facility, and the Micro Nano Research Facility at RMIT. Co-author Torben Daeneke received support from the RMIT Vice Chancellor's research fellow scheme.
More information: P. Atkin et al. Evolution of 2D tin oxides on the surface of molten tin, Chemical Communications (2018). DOI: 10.1039/C7CC09040D
Journal information: Chemical Communications
Provided by ARC Centres of Excellence