Novel bioactive steroid biosynthetic pathway in symbiotic fungi
A group of researchers from Graduate School of Pharmaceutical Sciences at The University of Tokyo and Institute of Traditional Chinese Medicine and Natural Products at Jinan University, identified the biosynthetic gene cluster for the furanosteroid demethoxyviridin, and deciphered its biosynthetic pathway.
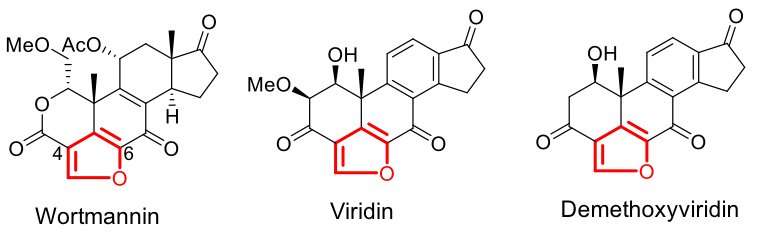
Furanosteroids, represented by wortmannin, viridin, and demethoxyviridin, are a special group of fungal-derived, highly-oxygenated steroids featured by an extra furan ring. They are nanomolar-potency inhibitors of phosphatidylinositol 3-kinase (PI3K), among which wortmannin has been developed as a commercial PI3K inhibitor widely used in various biological studies, exemplified by semisynthetic analogue of wortmannin, PX-866, tested in a Phase II clinical trial for treating cancers. The intriguing structures and excellent biological activities of furanosteroids have thus led to extensive efforts toward their total chemical synthesis over the past 20 years, and the stereoselective synthesis of wortmannin and (-)-viridin was finally achieved in 2017. However, as compared with the progress in chemical synthesis, the biosynthesis of these important molecules in fungi is poorly understood.
To identify the biosynthetic gene cluster of demethoxyviridin, the research group sequenced the whole genome of Nodulisporium sp. (a symbiotic fungus which produces demethoxyviridin), and identified a total of 103 cytochrome P450 monooxygenase genes in the genome. The CYP gene clusters in the genome could be potential targets, since demethoxyviridin possesses a highly oxygenated structure. Analyses of the relative localizations of these genes in the genome revealed twelve CYP clusters containing two or more CYP genes.
To determine the candidate gene cluster of demethoxyviridin, one CYP gene from each gene cluster was randomly selected and its expression was analyzed by reverse transcription PCR, under demethoxyviridin productive and non-productive conditions. As a result of CRISPR-Cas9-based gene disruption of the candidate genes, the group identified the gene cluster for demethoxyviridin production in this symbiotic fungus. Successive analyses by using Aspergillus oryzae heterologous gene expression system, and an in vitro enzymatic assay further confirmed each of the biosynthetic step and yielded the fourteen biosynthetic intermediates.
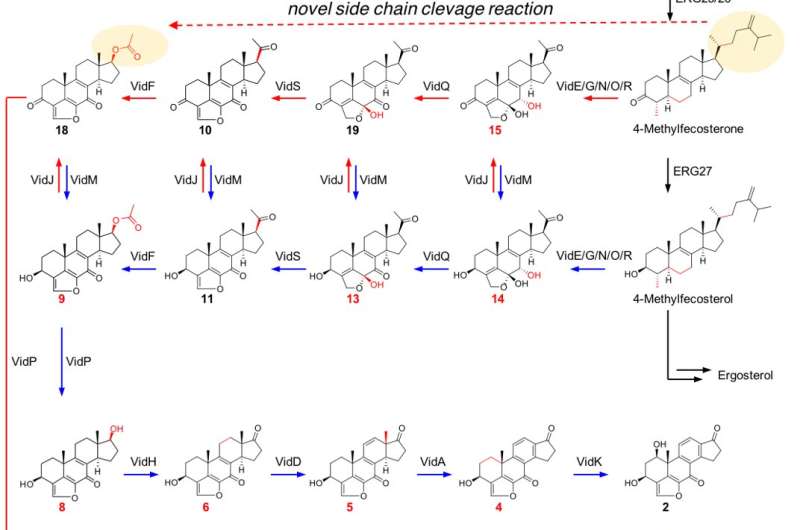
Structure-activity analyses of the intermediates revealed that the 3-keto group, the C1β-OH, and the aromatic ring C are important for PI3K inhibition. In addition, the in vitro studies revealed that the pregnane side-chain cleavage notably requires three enzymes, flavin-dependent Baeyer-Villiger monooxygenase, esterase, and dehydrogenase, in sharp contrast to the single CYP-mediated process in mammalian cells. Through extensive bioinformatic analyses, we revealed that these pregnane side-chain cleavage pathway is widely distributed in fungal genomes, and a conserved sterol metabolic pathway in the fungal kingdom.
This study set the stage to uncover the biosyntheses of other furanosteroids and expand the chemical diversity of pharmaceutically important furanosteroids by engineered biosynthesis. Because it also established the platform for genetic manipulation of symbiotic fungi, the knowledge obtained in this study will open the way to investigate the interaction between plants and symbiotic fungi.
More information: Gao-Qian Wang et al, Biosynthetic pathway for furanosteroid demethoxyviridin and identification of an unusual pregnane side-chain cleavage, Nature Communications (2018). DOI: 10.1038/s41467-018-04298-2
Journal information: Nature Communications
Provided by Japan Science and Technology Agency