View into the ultrahigh-vacuum chamber (catalyst sample in the middle). Credit: Vienna University of Technology
In chemistry, atoms can usually only affect their immediate neighborhood. At TU Vienna, a novel effect with astonishing long-range action has been discovered, which can make automotive catalytic converters more effective.
The taste of the chocolate cake's icing should not depend on whether it is served on a porcelain or a silver plate. Similarly, for chemical reactions on the surface of large precious metal grains, the substrate (the so-called support) should not play a crucial role. The catalytic grains often have a diameter spanning many thousands of atoms, and the support on which they rest should thus not affect chemical reactions on the other side far away from the interface—at least this was believed to date.
Experimental studies performed at TU Wien led to surprising findings. Chemical processes on palladium grains, which are also used for exhaust gas catalysts, changed significantly when they were placed on specific support materials—despite the fact that the material of the support is nearly inactive in the chemical reaction itself. This novel insight has now been published in the journal Nature Materials.
Toxic carbon monoxide
For vehicles using an internal combustion engine, toxic carbon monoxide (CO) must be converted into carbon dioxide (CO2). This is achieved by using catalysts containing palladium or platinum powder. "We have investigated chemical reactions on powder grains, which are often used in industrial catalysis," says Prof. Günther Rupprechter from the Institute of Materials Chemistry at TU Wien. "The precious metal grains have a diameter on the order of 100 micrometers—this is very large by nanotechnology standards, one can almost see them with the naked eye".
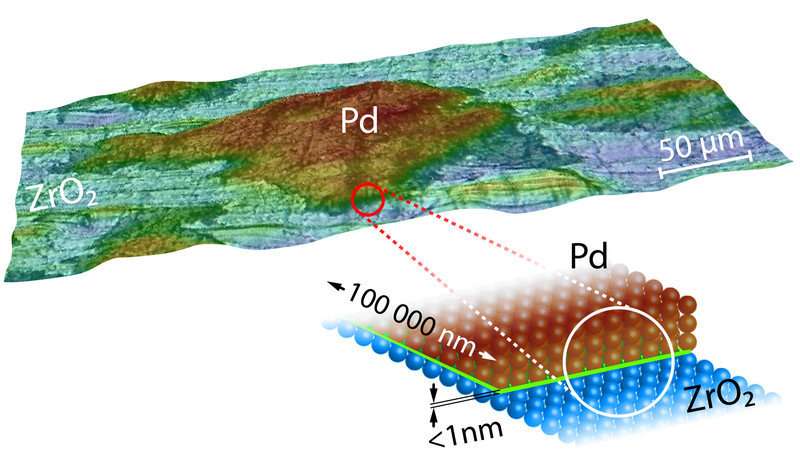
3-D-picture and a model drawing of a Pd-ZrO2 catalyst. The essential interface/borderline is colored green in the model. Credit: Vienna University of Technology
When the surface of the powder particles is covered by oxygen atoms, CO molecules react with them and are transformed to CO2, leaving empty sites (holes) in the oxygen layer. These sites must be quickly filled by other oxygen atoms to sustain catalysis. However, this is no longer the case when CO molecules fill these holes instead of oxygen. If this happens on a large scale, the catalyst surface is no longer covered by an oxygen layer but by a CO layer, and CO2 can thus not be formed anymore. This phenomenon is called "carbon monoxide poisoning", it deactivates the catalyst.
The support influences the entire grain
Whether this happens or not depends on the CO concentration in the exhaust gas supplied to the catalyst. However, as the current experiments show, the support material on which the palladium grains are placed is also crucial. "If the Palladium grains are placed on a surface of zirconium oxide or magnesium oxide, then poisoning of the catalyst occurs at much higher carbon monoxide concentrations," says Prof. Yuri Suchorski, the first author of the study. At first glance, this is very surprising for such large palladium grains. Why should the nature of the support have an effect on chemical reactions that take place on the surface of the entire metal grain? Why should the contact line between palladium grain and substrate, which is only a few tenths of a nanometer wide, influence the behaviour of palladium grains that are hundred thousand times larger?
This puzzle could finally be solved with the help of the special photoemission electron microscope at the Institute of Materials Chemistry at TU Wien. With this device, the spatial propagation of a catalytic reaction can be monitored in real time. "We can clearly observe that carbon monoxide poisoning always starts at the edge of a grain—exactly where it contacts the support," explains Prof. Yuri Suchorski. "From there, the "carbon monoxide poisoning" spreads like a Tsunami wave over the whole grain."
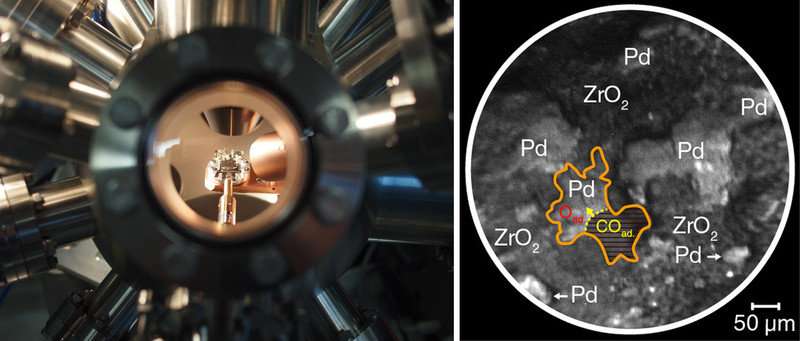
View into the ultrahigh-vacuum chamber (catalyst sample in the middle) and “in situ” PEEM image of a CO reaction front. Credit: Vienna University of Technology
Carbon monoxide attacks best at the border
It is mainly for geometrical reasons that the poisoning wave starts exactly there: the oxygen atoms at the border of the grain have fewer neighbouring oxygen atoms than those on the interior surface. When free sites opens up there, it is thus easier for a CO molecule to populate these sites than those sites somewhere in the middle of the free surface, where CO would easily react with other O atoms all around. In addition, it is not easy for other oxygen atoms to fill vacant areas at the border, since oxygen atoms always come in pairs, as O2 molecules. Therefore, to fill an empty site, O2 needs two free sites next to each other, and there is not much room for this at the border.
The borderline where the palladium grain is in direct contact with the support is therefore of great strategic importance—and exactly at this interface the support is able to influence the properties of the metal grain: "Calculations by our cooperation partners from the University of Barcelona show that the bond between the metal atoms of the grain and the protective oxygen layer is strengthened precisely at the borderline to the support," says Prof. Günther Rupprechter. The palladium atoms in intimate contact with the oxidic support can thus bind the oxygen stronger.
One may assume that this does not matter for metal sites far away from the border of the grain, because the support can only energetically influence atoms at the border—and these are only very few, as compared to the total number of atoms in the palladium grain. However, because carbon monoxide poisoning starts at the border, this effect is of great strategic importance. The metal-oxide border is in fact the "weak point" of the grain—and if this weak point is reinforced (the catalytic properties of metal atoms at the border are positively affected by the support), the entire micrometer-size catalyst grain is protected from carbon monoxide poisoning.
"Various oxide supports are already used in catalysts, but their exact role during catalysis in terms of CO poisoning has not yet been directly observed" says Prof. Günther Rupprechter. "With our methods, the ongoing process and its wave-like long-range effect were directly visualized for the first time, and this opens up promising new routes towards improved catalysts of the future".
More information: Yuri Suchorski et al. The role of metal/oxide interfaces for long-range metal particle activation during CO oxidation, Nature Materials (2018). DOI: 10.1038/s41563-018-0080-y
Journal information: Nature Materials
Provided by Vienna University of Technology