Protein analysis enables precise drug targeting
Researchers from MIPT and several U.S. and Chinese universities have solved the structure of one of the most important nervous system proteins in complex with a number of drug molecules. The discovery opens up opportunities for developing new medications with regulated action and fewer side effects. The paper was published in the journal Cell.
Many modern drugs target proteins since they are responsible for most of the physical and chemical reactions in a cell. Protein molecules also enable cells to communicate by transmitting signals between them. When a person falls ill, the harmony in cells is disrupted, so drugs are used to recover the balance by temporarily increasing or reducing the activity of proteins. Many of them serve similar functions and have almost identical structures, which means that one drug can affect several protein types. This ability of drugs to interact with multiple protein targets is called polypharmacology.
When the molecular approach to drug development was first introduced, a general notion among pharmacologists was that drug efficacy depended on how a particular medicine interacted with a given protein. The interaction with other protein types, however, was thought to only cause adverse side effects. So back then, the main aim of pharmacology was to maximize selectivity—that is, the ability of a drug to target only a specific type of proteins. The concept got a metaphorical name from Nobel Prize winner Paul Ehrlich who coined the term "magic bullets" to refer to such highly selective medications.
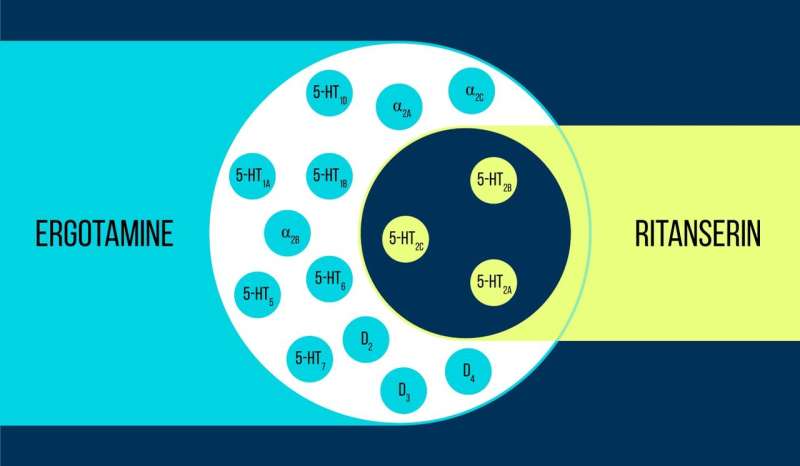
However, as it often happens, the simplest concept is not necessarily the most successful. Advances in computer technologies in chemistry and biology made it possible to produce extremely selective drugs that acted on only two or three close subtypes of a target protein. But they were not as effective as their low-selectivity analogs when treating complex disorders such as depression: The polypharmacological profiles of some drugs proved to be important for their beneficial effects. It turned out that polypharmacology does not necessarily cause adverse side effects, yet it is important to control which proteins are affected. The notion of a "magic bullet" was thus replaced by the "magic shotgun" concept, which emphasizes drugs with a desired effect on a particular combination of targets.
The purpose of this research was to identify the structural characteristics of proteins that would explain why some drugs act on them selectively and others do not. To carry out the study, the scientists used the 5-HT2c serotonin receptor—a signaling protein located in the cell membrane, which gets activated by serotonin to receive signals from neighboring cells. The receptor has a number of important features. First, it is already used as a validated target for anti-obesity medication, while also being a potential therapeutic target for several mental disorders. Second, it is targeted by a number of medications with a wide range of selectivities, making it possible to compare them. Thirdly, a human body has over 800 other receptors that are similar in structure to the 5-HT2c but serve different functions. That is why its non-selective antagonists often have a multitude of side effects.
Vsevolod Katritch, a visiting professor at MIPT, says, "We used two chemicals to work with the 5-HT2c serotonin receptor: ergotamine and ritanserin. Ergotamine is a nonselective agonist with a wide polypharmacological profile: It influences serotonin, dopamine, and adrenergic receptors. Ritanserin, by contrast, has a more narrow profile [figure 1] and is the 5-HT2c receptor-selective inverse agonist. Thus, the 5-HT2c atomic structures obtained in complex with ergotamine and ritanserin help not only explain the differences between active and inactive receptor states—which is in itself a considerable achievement—but also find out the reasons for such molecular selectivity."
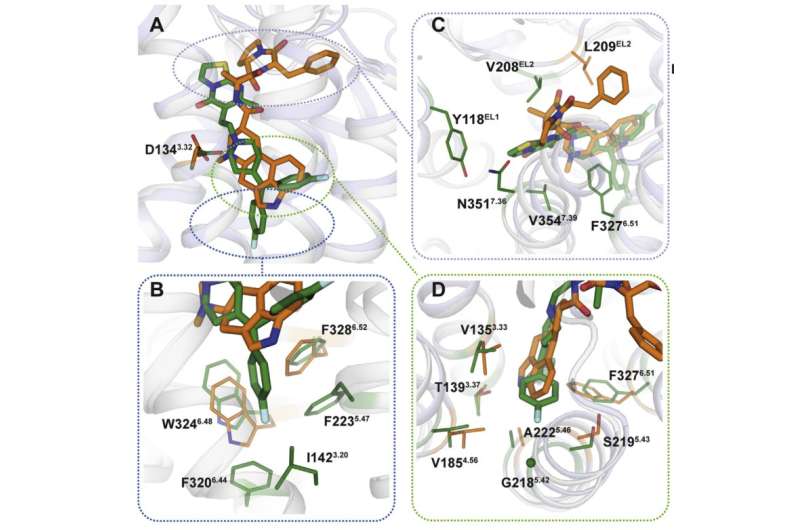
Using X-ray crystallography, the scientists obtained a 3-D model of proteins at the moment of their interaction with drugs. Predictably, the binding mechanisms of the medications were different (figure 2). The binding sites targeted by ergotamine are alike in many proteins, which explains the nonselectivity of the chemical. Ritanserin, on the other hand, interacts with the receptor differently and only acts on some of its fragments that are unique to a small group of proteins. Introducing several mutations that change these fragments into the 5HT2c receptor gene, the researchers noticed that the interaction with ritanserin became less effective—the fact which confirmed that those protein areas are the ones responsible for the chemical's selectivity.
Petr Popov, a researcher at MIPT's Laboratory of Structural Biology of G Protein-Coupled Receptors, says, "The main challenge in identifying receptor structures was obtaining a stable, genetically engineered construct that would be suitable for crystallization and that we could work with and study. Using the bioinformatic approach and machine learning methods, we have identified stabilizing point mutations for the 5HT2c receptor both in its active and inactive states."
Thus, the analysis of the structural characteristics of proteins in complex with drugs of varying selectivity proved to be effective. It can be used to control the set of targets and therefore both the direct and side effects of a medication during its development. Also, such medicines will benefit many patients thanks to improved therapeutic profiles that fight a variety of diseases and have fewer side effects.
More information: Yao Peng et al. 5-HT 2C Receptor Structures Reveal the Structural Basis of GPCR Polypharmacology, Cell (2018). DOI: 10.1016/j.cell.2018.01.001
Journal information: Cell
Provided by Moscow Institute of Physics and Technology