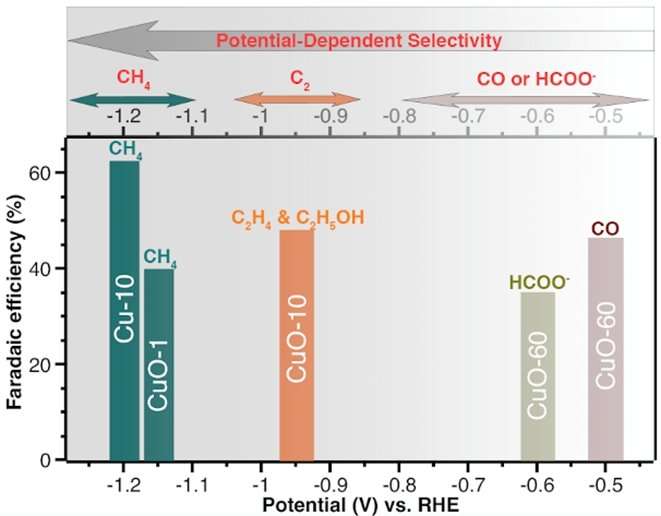
Figure shows that the selectivity of Cu catalysts (in producing specific carbon-based compounds) is determined by the applied voltage. Cu-10, CuO-1, CuO-10 and CuO-60 represent metallic copper and copper oxides with different surface morphology and roughness. Credit: National University of Singapore
NUS chemists have discovered key factors determining the selectivity of copper (Cu) catalysts for transforming carbon dioxide (CO2) and water into useful chemicals and fuels.
The electrochemical reduction of CO2 using renewable electricity is a promising technology for controlling CO2 emissions and producing high value-added chemicals. By supplying electrons to a Cu catalyst, carbon dioxide and water molecules which are attached to its surface can be transformed into useful molecules such as methane and ethylene. This is similar to the photosynthesis process in which CO2 and water are converted by plants into sugar.
Increasing the selectivity of CO2 reduction towards targeted products is one of the key challenges that need to be overcome in order to make the process more industrially viable. Previous research work to design selective copper catalysts has extensively focused on nano-structuring and defects engineering of their surfaces. In this work, the research team led by Prof YEO Boon Siang, Jason from the Department of Chemistry, NUS has discovered that the applied potential (electrical voltage) and magnitude of current, which have been largely overlooked, are key factors determining the selectivity of Cu catalysts in CO2 electroreduction reactions. The current produced at a particular electrical voltage is also largely dependent on the roughness of the Cu catalysts. By adjusting the applied voltage and the surface roughness of a Cu catalyst, CO2 and water can be made to favour the production of a specific carbon-based compound during the electrochemical reduction process.
The team's findings can enable the development of more selective catalysts for transforming CO2 into useful chemicals and fuels. For example, by tuning the surface roughness factor of a Cu sample to be 1.4 and the applied voltage to be -1.2 V versus reversible hydrogen electrode (a reference electrode), the selectivity of CO2 reduction to methane can be enhanced significantly to greater than 60% (see figure). This catalyst is among the most selective catalysts in the scientific community for methane formation. The performances of more than 20 previously-reported Cu-based catalysts were also analysed, and were shown to corroborate the team's findings.
Prof Yeo said, "We found that Cu catalysts derived from various precursors, may be similar in terms of chemical composition. However, their catalytic performance could be very different. Explanations such as the presence of steps, edges and defects on the catalysts are typically invoked to explain these phenomena. Our team discovered that the applied potential and mass transport of CO2, which are affected by currents, are also critical parameters affecting the selectivity Cu catalysts, and cannot be ignored."
More information: Dan Ren et al. The effects of currents and potentials on the selectivities of copper toward carbon dioxide electroreduction, Nature Communications (2018). DOI: 10.1038/s41467-018-03286-w
Journal information: Nature Communications
Provided by National University of Singapore