New carbon-dioxide-adsorbing crystals for biomedical materials that rely on shape-memory effect
Kyoto University scientists are one step closer to designing porous materials that can change and retain their shapes—a function known as shape-memory effect.
Shape-memory materials have applications in many fields. For example, they could be implanted in the body and then induced to change shape for a specific function, such as serving as the scaffold for bone tissue regeneration. The shape-memory effect is well documented in some materials, including ceramics and metal alloys. But it is rare and poorly understood in crystalline porous materials.
Now, Susumu Kitagawa of Kyoto University's Institute for Integrated Cell-Material Sciences and colleagues in Japan, Ireland and the U.S. have demonstrated a shape-memory effect in a flexible metal organic material—only the second such observation ever reported. They describe their findings in the journal Science Advances.
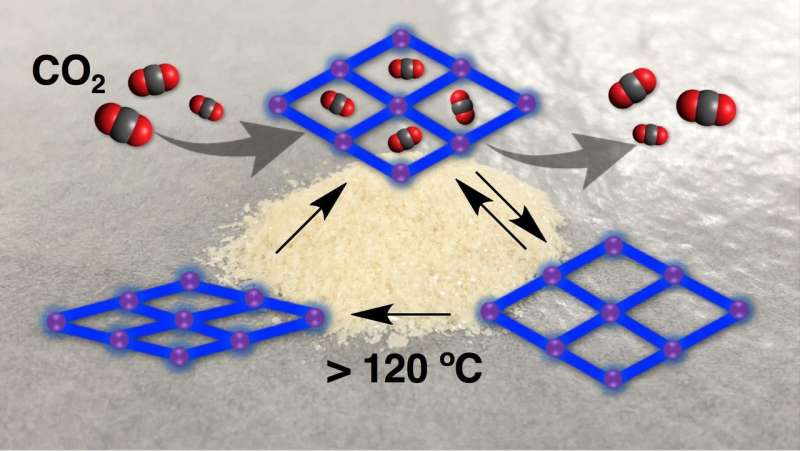
Crystals were made by dissolving a mixture of chemicals and zinc nitrate hexahydrate in a common solvent called dimethylformamide at 120°C for 24 hours. Using an X-ray technique called single-crystal X-ray diffraction, the team studied the crystals' structure. They found they were formed of slightly distorted paddlewheel-shaped lattices, which were made of central zinc ions linked to surrounding organic molecules. This 'alpha phase' of the crystal had 46 percent porosity, meaning that 46 percent of its volume was available for accepting new molecules; the property that makes porous materials suitable for a variety of applications.
When the team heated the alpha crystal at 130°C in a vacuum for 12 hours, the crystal became more dense, its lattices became more distorted, and its porosity was reduced to only 15 percent. They called this phase of the crystal its beta phase.
They then added carbon dioxide to the crystal at a temperature of -78°C. Carbon dioxide was adsorbed into the crystal's pores and the crystal's shape changed to less-distorted lattices than those in the beta phase. The available volume for accepting guest molecules increased to 34 percent. When the team added and removed carbon dioxide from the crystal over ten consecutive cycles, they found that it retained its shape. They called this phase of the crystal its 'shape-memory' gamma phase.
Adding nitrogen or carbon monoxide under varying temperatures also induced the transformation of the crystal from its beta to its gamma phase.
The team was able to revert the crystal's gamma phase back to its beta phase by heating it at 130°C in a vacuum for two hours. To revert to the alpha phase, the gamma phase of the crystal was soaked in dimethylformamide for five minutes.
The team's analyses of the crystal allowed them to have a better understanding of how its function changes along with structure. The researchers note their work could provide the basis for designing more examples of shape-memory porous materials.
More information: Mohana Shivanna et al. Readily accessible shape-memory effect in a porous interpenetrated coordination network, Science Advances (2018). DOI: 10.1126/sciadv.aaq1636
Journal information: Science Advances
Provided by Kyoto University


















