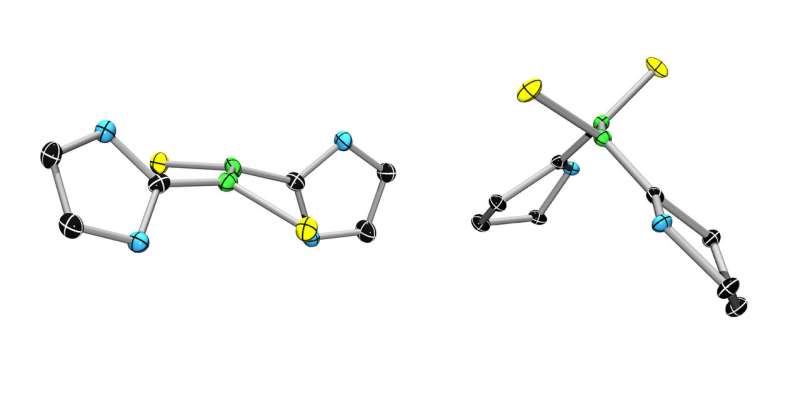
A conventional boron-boron double bond (left) and its extremely stable biradical relative. Credit: Dr. Rian Dewhurst
Boron has a range of uses, from laundry bleaches to heat-proof glass and ceramics. Chemists at Julius-Maximilians-Universität Würzburg (JMU) in Bavaria, Germany, have a particular interest in the chemistry of this element and have been researching the fundamental properties of boron for years. These researchers have now succeeded in twisting molecules with multiple bonds between boron atoms, leading to unusually stable biradicals.
Biradicals are usually highly reactive molecules. They are generated in energetic processes such as combustion and are normally so short-lived that they cannot be isolated or studied by traditional methods of chemical analysis.
The new biradicals prepared at the JMU are dramatically different, however. They are solid compounds and were found to be stable for weeks. "We now have model compounds in hand that we can study without having to rush," explains Prof. Holger Braunschweig from the Institute for Inorganic Chemistry. The results have been presented in the journal Nature Communications.
For a long time, chemists have attempted to twist, distort and rupture double bonds between atoms with only limited success. The JMU team has now made the dream of twisting a double bond by a full 90 degrees a reality. The Würzburg researchers had originally expected to obtain diborenes from their reactions. The products should have had double bonds between their boron atoms, as would normally be the case. Instead, they obtained molecules with double bonds between the atoms twisted by 90 degrees, and thereby completely broken.
The result of the experiments was the synthesis of unusually stable biradicals. "When a molecule is twisted against its will, it usually becomes less stable, and also more reactive," explains Julian Böhnke, doctoral student at the JMU and first author of the publication in Nature Communications. "The stability of the molecules is due to them being biradicals in their electronic ground state, despite their two unpaired electrons," says Braunschweig. "This structure was completely unexpected."
Applications of the molecules are still far off, according to Prof. Braunschweig. If they could be installed into a polymeric material, their use in organic electronics could become a possibility. However, Braunschweig says this is still a long way off. The next step for the JMU chemists is to test whether similarly stable biradicals can be prepared with double bonds between boron and carbon.
More information: Julian Böhnke et al, Isolation of diborenes and their 90°-twisted diradical congeners, Nature Communications (2018). DOI: 10.1038/s41467-018-02998-3
Journal information: Nature Communications
Provided by University of Würzburg