Making rusty polymers for energy storage
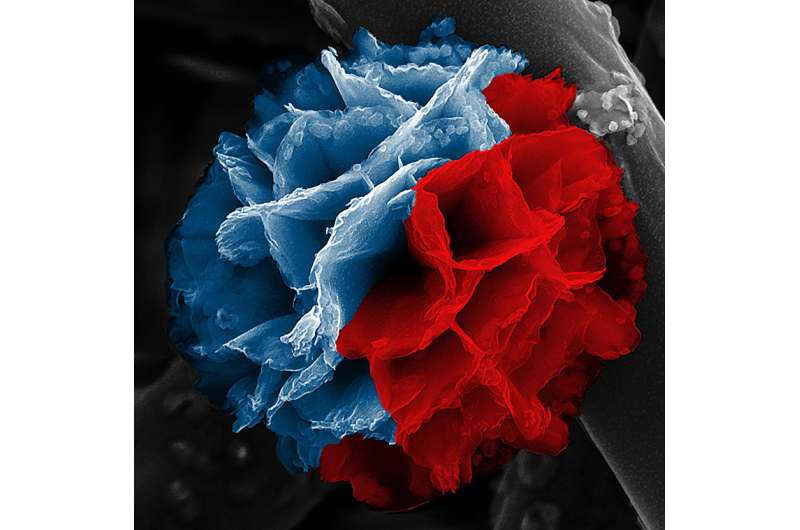
It's called a nanoflower, but if you could brush your cheek against its microscopic petals, you would find them cool, hard and … rusty.
Common rust forms the inner skeleton of these lovely and intricate nanostructures, while their outer layer is a kind of plastic.
Researchers at Washington University in St. Louis have developed a straightforward way to make this type of conducting polymer with high surface area that is likely to be useful for energy transfer and storage applications.
The new research is the cover story of the March 23 issue of ACS Applied Nano Materials.
"Rust will always pose a challenge in Earth's humid and oxygenated atmosphere," said Julio M. D'Arcy, assistant professor of chemistry in Arts & Sciences and a member of the university's Institute of Materials Science and Engineering. "Corrosion makes structures fragile and decreases the ability of components to function properly. But in our lab, we've learned how to control the growth of rust so that it can serve an important purpose."
Conducting polymers rely on a combination of organic and inorganic materials—usually a core of metal and a shell of plastic—made in a single batch.
D'Arcy and his team reported on a new technique that combines vapor-phase synthesis with solution-based hydrolysis to build three-dimensional nanoflowers, two-dimensional nanoplates and one-dimensional nanofibers.
This work advances the understanding of the chemical mechanisms involved with depositing the rust and forming the polymer, which will allow scientists to more easily manipulate and engineer the structures of the materials they make.
"As chemists, my students and I are fascinated by conducting polymers because we can control their structure during synthesis," D'Arcy said. "How much electricity the polymers conduct is a function of their chemical pathway and their number of charge carriers, both of which can be optimized during synthesis."
As for the nanoflowers, D'Arcy said he will be sowing some new seeds soon. There are 16 stable phases of rust, all with different morphologies at the nanoscale—enough for a whole rusted garden.
More information: Hongmin Wang et al. Metal Oxide-Assisted PEDOT Nanostructures via Hydrolysis-Assisted Vapor-Phase Polymerization for Energy Storage, ACS Applied Nano Materials (2018). DOI: 10.1021/acsanm.7b00382
Provided by Washington University in St. Louis