Stepwise hydride transfer in the biosynthesis of chlorophyll
Hydride transfer is an important reaction for chemistry (e.g., fuel cells), as well as biology (e.g., respiratory chain and photosynthesis). Often, one partial reaction involves the transfer of a hydride ion (H−). But does this hydride transfer involve one step or several individual steps? In the journal Angewandte Chemie, scientists have now provided the first proof of stepwise hydride transfer in a biological system.
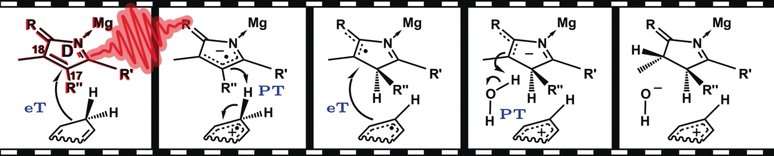
An important step in the biosynthesis of chlorophyll is the light-dependent hydrogenation of protochlorophyllide to chlorophyllide. This involves the reduction of a double bond between carbon atoms 17 and 18 in this complex ring system to a single bond as both carbon atoms bind to an additional hydrogen atom. This step is catalyzed by the enzyme protochlorophyllide oxireductase and requires irradiation with light. Technically speaking, however, this reaction does not add one hydrogen atom to each carbon. Instead, there is first addition of a hydride ion (H–) to C17 and then addition of a proton (H+) to C18. The first partial reaction, the hydride transfer, requires the cofactor nicotinamide adenine dinucleotide phosphate (NADPH). NADPH serves as a source for two electrons and a proton (H+), the equivalent of a hydride anion, H–.
Hydride transfer reactions play a key role in many biological systems. However, their mechanism is still disputed. Do the three elementary steps—transfer of an electron, a proton, and another electron from NADPH to the substrate—occur simultaneously, or stepwise?
Because of the short lifetime of the intermediates, direct proof of a stepwise mechanism has not previously been possible. Light-dependent reactions—such as the hydrogenation that occurs in the biosynthesis of chlorophyll—that can be triggered by a short laser pulse have solved this problem. By using time-resolved absorption and emission spectroscopy, researchers working with Roger J. Kutta and Nigel S. Scrutton at the University of Manchester (UK) have been able to characterize the mechanism of this hydride transfer.
In addition to excited states of protochlorophyllide, the researchers were able to resolve three discrete intermediates that are consistent with a partially stepwise mechanism: an initial electron transfer from NADPH to protochlorophyllide that has been excited (to the singlet state) by light is followed by the coupled transfer of a proton and an electron. As expected, the final step is transfer of the second proton.
Interestingly, the researchers found different intermediates for the wild type of the enzyme and a mutated version (C226S): While the initial hydride binds to C17 in the wild type, it is transferred to C18 in the mutant version. However, the end result is the same chlorophyllide stereoisomer.
The insights gained from these experiments provide a deeper understanding of how light energy can be used for chemical reactions involving hydrogen transfer, particularly with regard to the design of light-activated catalysts.
More information: Nataliya Archipowa et al. Stepwise Hydride Transfer in a Biological System: Insights into the Reaction Mechanism of the Light-Dependent Protochlorophyllide Oxidoreductase, Angewandte Chemie International Edition (2018). DOI: 10.1002/anie.201712729
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Wiley