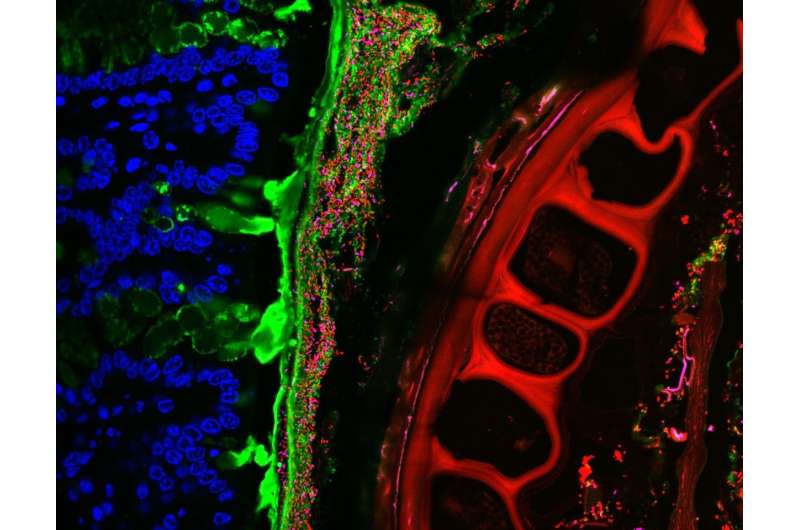
This distal colon of a mouse shows the mouse epithelial nuclei (blue), the mucus separating the epithelium and digesta (green), and a piece of food fiber (large structure in red). Bacteria are labeled in red and magenta. Credit: Carolina Tropini/Stanford University
When bacteria enter the body, they have a great deal to overcome to colonize the colon. First, they must survive harsh environments with very few salts without bursting (unlike human blood cells within water). Then, they navigate through saliva enzymes and stomach acid, bypass our immune systems within the small intestine, switch from being exposed to oxygen to having none at all, and hang on so that they're not flushed away by the gut's constant outward flow.
During the 62nd Biophysical Society Annual Meeting, held Feb. 17-21, in San Francisco, California, Carolina Tropini, a postdoctoral research fellow at Stanford University, presented her work exploring how bacteria living in the gut respond to common changes within their habitat.
How are microbes living in and on our bodies intimately linked to our health? "They make compounds we're unable to produce, and the molecules they produce are readily absorbed into our blood system—no differently than a drug we'd take by mouth," said Tropini. "Changes in the bacteria that live in our gut are linked to diseases ranging from colon cancers to neurological disorders. So, my goal is to study how communities of bacteria respond to and change the physical environment of the host in health and disease."
First, Tropini administers a common over-the-counter drug to mice to change their gut environment. Then, she measures which bacterial species survive the change using DNA sequencing techniques. "The idea is that we can tell which bacterium [the DNA] comes from by sequencing only a small region of a very conserved gene, which differs slightly between species," Tropini explained.
Tropini measures how the gut environment has changed and recreates a simplified version of it in the lab; the number of bacteria within the new habitat; and the physiological response of the bacteria—if they grow faster or slower, or produce different proteins.
What were the results? "In the short term, bacteria are impressively able to counteract changes ... but in a heavily competitive environment any disadvantage [or change in environment] can play a major role in changing the bacterial makeup of gut flora," Tropini said. "Bacterial species that make up a large fraction—even up to half—of the gut community may go extinct when the environment is altered even slightly, because they aren't competitive against all of the other species in this new environment."
What does this say about health implications? While the implications of these changes aren't entirely clear, "bacterial extinctions are hard to compensate for and may leave us with a community of gut microbes with less functionality," Tropini said. "By understanding how bacterial communities respond to common changes within their physical environment, we can design probiotics and therapies to directly target weaker parts of the community to make them more resilient."
More information: 1630-Pos, Board B539 "Mechanical perturbations to the gut microbiota" is authored by Carolina Tropini, Justin Sonnenburg, KC Huang and Katharine Ng. It was displayed Monday, Feb. 19, 2018, in the South Hall ABC of the Moscone Center, South. Abstract: plan.core-apps.com/bpsam2018/a … 1290aae09439cc46a7f2
Provided by Biophysical Society