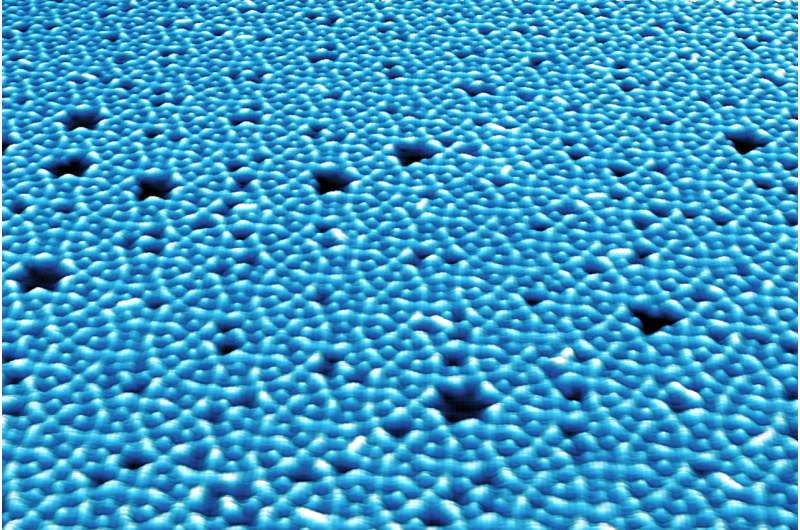
The new building block (left, red outline) comprises two modified starting molecules connected to each other by a silver atom (blue). This leads to complex, semiregular tessellations (right, microscope image). Credit: Klappenberger and Zhang / TUM
An international team of researchers lead by the Technical University of Munich (TUM) has discovered a reaction path that produces exotic layers with semiregular structures. These kinds of materials are interesting because they frequently possess extraordinary properties. In the process, simple organic molecules are converted to larger units which form the complex, semiregular patterns. With experiments at BESSY II at Helmholtz-Zentrum Berlin this could be observed in detail.
Only a few basic geometric shapes lend themselves to covering a surface without overlaps or gaps using uniformly shaped tiles: triangles, rectangles and hexagons. Considerably more and significantly more complex regular patterns are possible with two or more tile shapes. These are so-called Archimedean tessellations or tilings.
Materials can also exhibit tiling characteristics. These structures are often associated with very special properties, for example unusual electrical conductivity, special light reflectivity or extreme mechanical strength. But, producing such materials is difficult. It requires large molecular building blocks that are not compatible with traditional manufacturing processes.
Complex tessellations through self-organization
An international team led by Professors Florian Klappenberger and Johannes Barth at the Chair of Experimental Physics of TUM, as well as Professor Mario Ruben at the Karlsruhe Institute of Technology, have now made a breakthrough in a class of supramolecular networks: They got organic molecules to combine into larger building blocks with a complex tiling formed in a self-organized manner.
As a starting compound, they used ethynyl iodophenanthrene, an easy to handle organic molecule comprising three coupled carbon rings with an iodine and an alkyne end. On a silver substrate, this molecule forms a regular network with large hexagonal meshes.
Heat treatment then sets a series of chemical processes in motion, producing a novel, significantly larger building block which then forms a complex layer with small hexagonal, rectangular and triangular pores virtually automatically and self-organized. In the language of geometry this pattern is referred to as a semiregular 3.4.6.4 tessellation.
Atom economy through by-product recycling
"The scanning tunnel microscopy measurements we conducted at TUM show clearly that the molecular reorganization involves many reactions that would normally result in numerous by-products. In this case, however, the by-products are recycled, meaning that the overall process runs with great economy of atoms—nearly one hundred percent recovery—to arrive at the desired end-product," explains Prof. Klappenberger.
The researchers uncovered precisely how this happens in further experiments. "Using X-ray spectroscopy measurements at the electron storage ring BESSY II of the Helmholtz-Zentrum Berlin, we were able to decipher how iodine splits from the starting product, hydrogen atoms move to new positions and the alkyne groups capture the silver atom," explains lead author Yi-Qi Zhang.
By way of the silver atom, two starting building blocks bind together to a new, larger building block. These new building blocks then form the observed complex pore structure.
"We have discovered a completely new approach to produce complex materials from simple organic building blocks," summarizes Klappenberger. "This is important for the ability to synthesize materials with specific novel and extreme characteristics. These results also contribute to better understanding the spontaneous appearance (emergence) of complexity in chemical and biological systems."
The study is published in Nature Chemistry.
More information: Complex supramolecular interfacial tessellation through convergent multi-step reaction of a dissymmetric simple organic precursor, nature.com/articles/doi:10.1038/nchem.2924
Journal information: Nature Chemistry
Provided by Helmholtz Association of German Research Centres