January 10, 2018 report
Finding liquid water's coldest temperature and its singularity

Two teams of researchers working independently of one another have discovered some remarkable features of liquid water—it can be chilled to −42.55°C and it appears to have what is described as a singularity. The first team, made up of members from across Europe, conducted experiments designed to find the lowest temperature at which liquid water can exist. They have published their results in the journal Physical Review Letters. The second team, with members from Sweden, Korea and Japan sought to learn more about the attributes of liquid water when chilled to very low temperatures. They have published their results in the journal Science. Paola Gallo with Università Roma Tre and Eugene Stanley with Boston University offer a Perspective piece on the work done by the two teams in the same Science issue.
While it is true that liquid water normally becomes a solid at 0°C, it is also true that under certain circumstances, liquid water can be much colder than that, such as when it is chilled very quickly—a fast measuring system can take the temperature of the water before it has time to form crystals. But just how cold can it get? Prior theory has suggested the limit is likely −40°C. I this new effort, the researchers found it could go slightly colder than that by injecting very tiny droplets into a vacuum chamber. As the droplets moved through the chamber, some of the water evaporated, causing the temperature of the drop to fall. The team used lasers to measure the change in diameter of the drops to calculate their temperature, and found the new low record of −42.55°C.
Liquid water also has another strange property, according to the researchers with the second effort—the ability to exist in two different liquid states when chilled and to jump between them at a given point, which the team called its singularity. They used a similar setup to the first group, injecting water droplets into a vacuum chamber and shooting them with a laser to measure their temperature, but they found something new. Different parts of the droplets had different densities and sometimes the molecules in the drops seemed to have difficulty deciding which state to occupy, and so moved back and forth, suggesting a singularity state.
More information: 1. Claudia Goy et al. Shrinking of Rapidly Evaporating Water Microdroplets Reveals their Extreme Supercooling, Physical Review Letters (2018). DOI: 10.1103/PhysRevLett.120.015501 , On Arxiv: arxiv.org/abs/1711.02412
Abstract
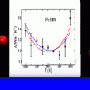
The fast evaporative cooling of micrometer-sized water droplets in vacuum offers the appealing possibility to investigate supercooled water - below the melting point but still a liquid - at temperatures far beyond the state-of-the-art. However, it is challenging to obtain a reliable value of the droplet temperature under such extreme experimental conditions. Here, the observation of morphology-dependent resonances in the Raman scattering from a stream of perfectly uniform water droplets has allowed us to measure with an absolute precision of better than 0.2% the variation in droplet size resulting from evaporative mass losses. This finding proved crucial to an unambiguous determination of the droplet temperature. In particular, a fraction of water droplets with initial diameter of 6379±12 nm were found to remain liquid down to 230.6±0.6 K. Our results question temperature estimates reported recently for larger supercooled water droplets, and provide valuable information on the hydrogen-bond network in liquid water in the hard-to-access deeply supercooled regime.
2. Kyung Hwan Kim et al. Maxima in the thermodynamic response and correlation functions of deeply supercooled water, Science (2017). DOI: 10.1126/science.aap8269
Abstract
Femtosecond x-ray laser pulses were used to probe micrometer-sized water droplets that were cooled down to 227 kelvin in vacuum. Isothermal compressibility and correlation length were extracted from x-ray scattering at the low–momentum transfer region. The temperature dependence of these thermodynamic response and correlation functions shows maxima at 229 kelvin for water and 233 kelvin for heavy water. In addition, we observed that the liquids undergo the fastest growth of tetrahedral structures at similar temperatures. These observations point to the existence of a Widom line, defined as the locus of maximum correlation length emanating from a critical point at positive pressures in the deeply supercooled regime. The difference in the maximum value of the isothermal compressibility between the two isotopes shows the importance of nuclear quantum effects.
Journal information: Physical Review Letters , Science , arXiv
© 2018 Phys.org