Cancer's gene-determined 'immune landscape' dictates progression of prostate tumors
The field of immunotherapy - the harnessing of patients' own immune systems to fend off cancer - is revolutionizing cancer treatment today. However, clinical trials often show marked improvements in only small subsets of patients, suggesting that as-yet unidentified variations among tumors result in distinct paths of disease progression and response to therapy.
Now, researchers at the Cancer Center at Beth Israel Deaconess Medical Center (BIDMC) have demonstrated that genetic variations driving prostate cancer determine the composition of the immune cells that have been found to infiltrate primary prostate tumors. These immune cells, in turn, dictate tumor progression and response to treatment. The data, published in Nature Medicine, suggest that profiling patients' tumors based on this new information could lead to more successful clinical trials and tailored therapies for patients.
"We observed that specific genetic events resulted in striking differences in the composition of immune cells present in and around the tumor - results with important therapeutic implications," said senior author Pier Paolo Pandolfi, MD, PhD, Director of the Cancer Center and Cancer Research Institute at BIDMC. "Our data may be especially relevant for tailoring immunological therapies and for identifying responsive-patient population."
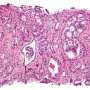
The third leading cause of cancer-related death in U.S. men, prostate cancer, is linked to a number of diverse genetic mutations that drive the disease. For example, the loss of the tumor suppressor gene PTEN is a frequent event in prostate cancer and is well known to promote the disease in combinations with a plethora of other mutations. Researchers also know that the tumor's microenvironment - the blood vessels, immune cells, signaling molecules and other factors that surround the tumor - plays an important role in tumor progression and response to therapy.
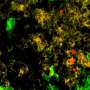
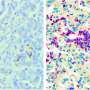
Pandolfi's team - including lead author, Marco Bezzi, a post-doctoral fellow in Pandolfi's lab - engineered mice models to represent four distinct known genetic variations of human prostate cancer. The models lacked either Pten alone or in combination with other genetic alterations known to drive the disease. When the team analyzed the tumors from these mice, they saw profound differences in the types and relative numbers of the immune cells that had accumulated in and around the tumor, what they call the tumors' "immune landscape".
For example, specific immune landscapes tumors from the genetic model lacking both Pten and the tumor suppressor gene called Trp53 demonstrated an increased accumulation of myeloid cells, the immune cells that mediate immunosuppression. In stark contrast, tumors from the genetic model lacking Pten and a different tumor suppressor gene called PML lacked intratumoral immune infiltration; that is, the researchers observed no immune cells at all in these tumors, which the scientists dubbed "cold," or "immune-deserts." All four mouse models analyzed presented very distinctive immune landscapes and these differences were maintained and exacerbated over time.
The research team also demonstrated that these differences in immune cell composition were directly dictated by the tumors themselves because of their genetic variations. Different tumors, they observed, secreted distinct chemical attractants, which in turn recruited - or didn't recruit, in the case of the immune-desert tumors - different immune cell types into the tumor. Pandolfi and colleagues further demonstrated that these differences hold true in human prostate cancer. Critically, the immune cells recruited to the tumors were found to be essential in supporting the growth and progression of these tumors.
"We observed that when present, these infiltrating immune cells were required for the tumor to thrive and found therapies to block their recruitment to be effective," said Bezzi. "On the other hand, the cancer genotype characterized by the so-called 'immune desert' phenotype, did not respond to such therapies. On this basis, we can predict the tumor response to immunotherapies and tailor treatment modalities to effectively impact tumors that are otherwise extremely aggressive," he said.
Thus, because immune cells interact with and also affect tumor response to therapy, these findings may be especially relevant for the development of more precise and effective combinations of immunotherapies and targeted therapies on the basis of the cancer genetic makeup.
"These profound differences in immunological landscapes among various cancer genotypes further highlight the need to thoroughly investigate and integrate genotypes and immune-phenotypes in the context of exploratory cancer treatments in both preclinical and clinical settings," said Pandolfi.
More information: Diverse genetic-driven immune landscapes dictate tumor progression through distinct mechanisms, Nature Medicine (2018). nature.com/articles/doi:10.1038/nm.4463