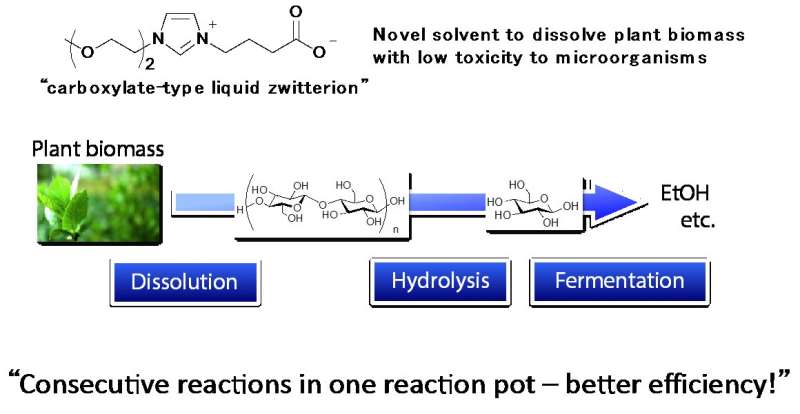
After dissolving plant biomass by the novel solvent, carboxylate-type liquid zwitterion, hydrolysis and fermentation were consecutively carried out in one reaction pot for conversion into ethanol. Credit: Kanazawa University
Compared to first-generation biofuels produced from food crops, production of second-generation biofuels for daily use is an urgent issue. In this study, researchers developed a novel carboxylate-type liquid zwitterion as a solvent of biomass, which could dissolve cellulose with very low toxicity to microorganisms. Use of this novel solvent enables significant reduction of energy cost for ethanol production from non-food biomass. Thus, second-generation biofuel ethanol production is in sight of practical implementation.
Ethanol is produced from food crops like maize, and thus poses a threat to food supply. It is therefore necessary to produce ethanol from non-food biomass like weeds, waste paper, etc. Solvents needed for the production of second-generation biofuel ethanol are highly toxic to microorganisms. Complicated processes are necessary to remove such highly toxic solvents, such as washing with water, separation by centrifugation and compression. As a result, the energy recovered in this ethanol is less than that required to produce it, i.e., there is a negative energy balance and a greater load on the environment. It was considered impossible to solve this problem, since a harsh solvent was needed to break down recalcitrant plant materials like cellulose, while such a harsh solvent would kill microorganisms that play essential roles in the fermentation necessary for producing ethanol.
In the present study, researchers of Kanazawa University, Japan, succeeded in reducing the toxicity to microorganisms by developing a novel solvent, a carboxylate-type liquid zwitterion for dissolving biomass cellulose (Figure 1). The EC50, the concentration of a substance that reduces the growth of Escherichia coli to 50 percent, was found to be 158 g/L for the newly developed carboxylate-type liquid zwitterion, whereas the EC50 of ionic liquid, one of the conventional solvents of cellulose, was 9 g/L. This indicates that the novel carboxylate-type liquid zwitterion shows 17-fold lower toxicity than the ionic liquid.
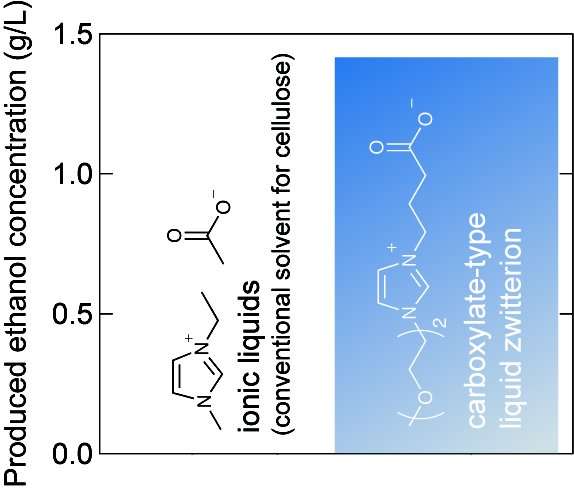
With the ionic liquid (left), no ethanol production was observed while with the carboxylate-type liquid zwitterion (right), ethanol production was observed. Credit: Kanazawa University
With Escherichia coli that can produce ethanol, fermentation ability was almost maximal in 0.5 mol/L carboxylate-type liquid zwitterion with a final ethanol concentration of 21 g/L. On the other hand, the same experiment with the ionic liquid produced only 1 g/L ethanol. Thus, fermentation in the presence of the carboxylate-type liquid zwitterion produced 21 times more ethanol than that using the ionic liquid.
In another experiment, bagasse was used as a starting plant biomass for ethanol production without washing/separation processes. Fermentation in the presence of the carboxylate-type liquid zwitterion produced 1.4 g/L ethanol, while no ethanol was obtained with the ionic liquid due to its high toxicity (Figure 2).
With these experimental results, it is shown that by using the carboxylate-type liquid zwitterion, plant biomass could be converted into ethanol in a single reaction pot without washing/separation processes. This represents a big step forward in the production and use of second-generation biofuel ethanol through reducing large amounts of energy input.
Besides first-generation and second-generation biofuel ethanol, a third-generation biofuel, a kind of oil, may be made from some algal species. In order to obtain such a third-generation biofuel from algae, polysaccharides like cellulose, which are main components of cell walls, have to be dissolved. The energy efficiency would be much increased if dissolved polysaccharides could be converted into ethanol. Further development of our current study would significantly contribute to the production of not only second-generation but also third-generation biofuel ethanol.
More information: Kosuke Kuroda et al, Design of Wall-Destructive but Membrane-Compatible Solvents, Journal of the American Chemical Society (2017). DOI: 10.1021/jacs.7b08914
Journal information: Journal of the American Chemical Society
Provided by Kanazawa University