Researchers get first complete look at protein behind sense of touch
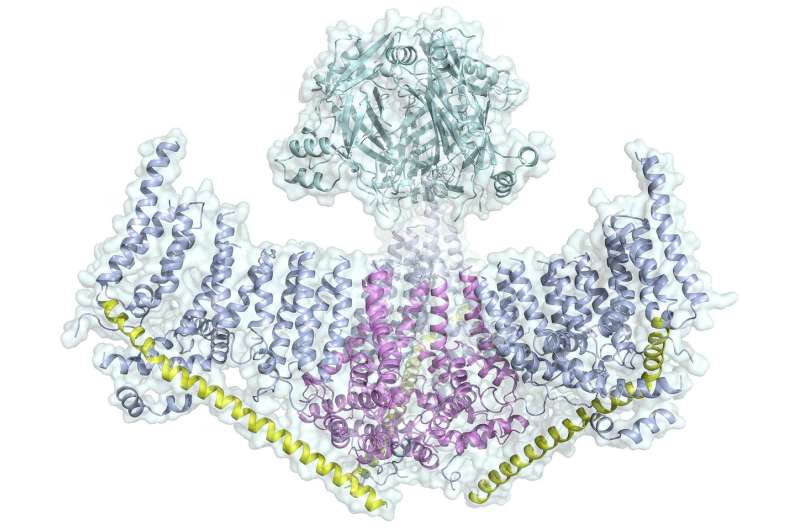
Scientists at The Scripps Research Institute (TSRI) have solved the mystery of the structure of Piezo1, a member of a family of proteins that convert physical stimuli such as touch or blood flow into chemical signals. The findings, published today in the journal Nature, point the way to targeting diseases where Piezo1 is mutated, such as dehydrated hereditary stomatocytosis and congenital lymphedema.
"This structure provides a fundamental understanding of how proteins sense mechanical force, and will shed light on regions within Piezo1 that can be targeted using small molecules or antibodies," says Ardem Patapoutian, PhD, a TSRI professor and Howard Hughes Medical Institute investigator, who co-led the new study with TSRI Professor Andrew Ward, PhD.
Piezo1, and the closely related Piezo2 protein, were discovered in the Patapoutian Lab in 2010. Piezos are known as an ion channel—they let ions through a pore in response to a mechanical stimulus (aka touch). These proteins have been found in disparate cell types and are already shown to be key players in sensory neurobiology and vascular physiology.
Until now, however, researchers didn't know how Piezo1 looked at the structural level, i.e., how its parts come together.
The new study, carried out with a high-resolution imaging technique called cryo-electron microscopy (cryoEM), shows that Piezo1 is made up of three curved "blades" circling a central pore. The researchers believe these blades move in response to mechanical force, which opens and closes the pore to let ions through to send the signal to communicate touch.
A beam-like structure serves as the backbone for each blade. An "anchor domain" surrounds the pore where the blades meet the middle.
"It is a beautiful structure," says Patapoutian. "The features identified in this study give us tantalizing clues on how this ion channel would sense and respond to membrane tension."
Ward says the Piezo1 structure is unique because it appears to be an "all-in-one" protein, meaning it does not need to connect with other proteins or cell structures to do its job transmitting a signal.
The next step in this research is to examine the overall architecture of Piezo1 and determine how each piece works. Scientists hope to look at this protein in different conformations besides the closed conformation seen in the current study.
"This gives us a good opportunity to investigate how force sensation, or transduction, really works," says Kei Saotome, PhD, TSRI research associate and first author of the new study.
More information: Kei Saotome et al, Structure of the mechanically activated ion channel Piezo1, Nature (2017). DOI: 10.1038/nature25453
Journal information: Nature
Provided by The Scripps Research Institute