Researchers identify the inner workings of cellular calcium pumps

For the first time, researchers have observed the ion pumps that activate cell transport and signal systems. This ensures the function of biomolecular mechanisms.
Each calcium pump measures only a few nanometres in each direction, and resides in the cell membranes. But despite its diminutive size, it is crucial to life. This pump is the reason that our muscles can contract, and that neurons can send signals. If the tiny pump stopped working, cells would stop communicating. This is why cells use so much of their energy – about a fourth of the body's fuel, known as ATP – to keep the pumps running.
There are many things that we still do not know about the structure and function of this vital pump. Knowledge about the pump is essential to understand energy balance and other important functions in the body.
A Danish research group has just published a new study that for the first time shows how the pump functions at the level of a single molecule, and how it ensures that ions are pumped in the right direction. In other words, how the pump works as a molecular one-way street. The discovery has just been published in the prestigious journal Nature.
"This work represents the next step of a profound and important quest to understand the pump's atomic structure and function. We are now one step closer to understanding how the ion pumps ensure the functions of the cells. We have characterised how it pumps ions out of the cell at an unprecedented level of detail. The importance of such basic knowledge of biophysical processes can only be underestimated. It will have a great influence on our understanding of the processes of life and, in time, on disease treatment," says Professor Poul Nissen. Professor Nissen is one of the world's leading experts on this family of pumps and a co-author of the paper.
The molecular backstop
To some extent, the story began in the 1950s, when Professor Jens Christian Skou did his pioneering work at Aarhus University, which uncovered the pumping functions in our cells. The calcium pump is a close cousin of the sodium-potassium pump Skou worked on, and they use a similar pumping mechanism. Skou's work earned him a Nobel Prize in chemistry in 1997. Since then, numerous researchers have studied the mechanism and function of these pumps, including many at the Centre for Membrane Pumps in Cells and Disease – PUMPkin – at Aarhus University.
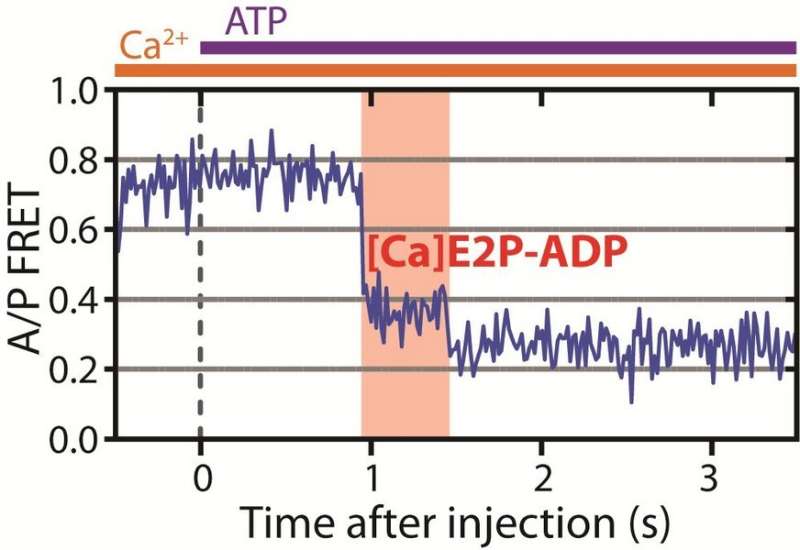
A key insight in the new publication concerns the one-way nature of ion transport. Previously, it was assumed that the one-way nature of the pump arose in the cleavage of the energy-rich molecule ATP. The hypothesis was that when ATP was cleaved, the pump could not backtrack and reform ATP. That turned out to be incorrect.
"We have identified a new closed state in the pumping cycle, which the pump can only enter if the calcium ion comes from the intracellular fluids and the pump has cleaved ATP. It cannot reach this state if the ion comes from the cell's surroundings. When calcium is released from this state, it is the 'point of no return.' This is the mechanism that explains that the pump works as a pump and not just a passive channel. This really unique insight is based on highly advanced experiments. These experiments enable us to directly see the pump doing its job for the first time," says Postdoctoral Fellow Mateusz Dyla, the first author of the new paper.
The calcium pump needs energy, which it gets from cleaving a molecule of ATP. The energy released is converted into the work of the pump. This explains how large concentration gradients build up between the inside and the outside of the cell. The concentration difference can be more than 10,000-fold, and this large difference is essential to the communication between cells such as in nerve signaling.
Smoke and mirrors
The reason the experiments are so complex is pretty clear. The pump is so small that it cannot be imaged directly in a light microscope. So far, and with great difficulty, researchers have created molecular models of stable states of the pump using a technique known as X-ray crystallography. This is analogous to a stop-motion movie. The scientists have jokingly referred to their visualisation of the pump's movement between these states as 'Pump Fiction.' The new study, which has been five years in the making, moves the visualisation from stop motion to live images of the functional movements of the pump. Technical improvement in microscopic techniques has allowed the new state to be observed.
The technique is known as single-molecule fluorescence spectroscopy, and uses a phenomenon known as Förster resonance energy transfer, in short FRET. Here, intense laser light and ultra-sensitive cameras are combined to allow the direct observation of a single molecule through the tiny amount of light each molecule emits.
The research group has taken advantage of a calcium pump from the bacterium Listeria, which was prepared for the studies through protein engineering. The engineering of the protein alone took several years to complete.
In the FRET experiments, two dye molecules are attached to the protein, which is then illuminated by laser light. One dye, the donor, absorbs the laser light and either emits it with a characteristic colour, or alternatively transfers the energy to the other dye, the acceptor. This will then emit light with another colour. Light will thus be emitted from the two dyes, and scientists can measure the distance between the two dyes by measuring how much light is emitted of each colour. Because the dyes have been carefully inserted in two specific positions in the pump, these distance changes track the pumping movements of the pump.
The single molecule technique enabled the new discoveries, as explained by Fellow Magnus Kjærgaard, Aarhus Institute of Advanced Studies (AIAS), who also contributed to the discovery.
"We have moved from 'Pump Fiction' to 'Pump Live'. Previously, we always recorded the signals from many molecules at the same time, which blurs the movements. Using single-molecule FRET techniques, we can focus on one molecule at a time, which allows us to observe the structural changes directly. This provides us with a video of the pump in action with fewer gaps. Our Pump Fiction movie initially got its name because we knew the transitions between the different states of the cycle were fictional, and that there could be additional insights hiding in the gaps between the known states. We have now demonstrated this in abundance, and at the same time revealed critical new insights into how the pump works," he says.
Besides adding to our knowledge of the basic processes of life, the understanding of these pumps may also have practical applications. Mutations in the pumps can cause defects in brain cells, and this can cause neurological disorders such as migraine, temporary paralysis or neurodegenerative disorders.
The mechanisms of these ion pumps are thus pivotal to understanding the errors in the pump, especially with a view to developing new drugs targeting the pump.
"We haven't yet reached the stage where we can transfer our ion pump research into treatment of disease. However, the new insights have led to ideas that may be used to develop treatment of defects in neuronal signalling, for example. But this is work for the future. Right now, there is every reason to celebrate the revelation of the intimate details of one of the most important enzymes of life. The work has built on great collaborations here at the university, and with researchers in the USA. We have already started new exciting collaborations that will allow us to take the next steps," says Professor Poul Nissen.
More information: Mateusz Dyla et al. Dynamics of P-type ATPase transport revealed by single-molecule FRET, Nature (2017). DOI: 10.1038/nature24296
Journal information: Nature
Provided by Aarhus University