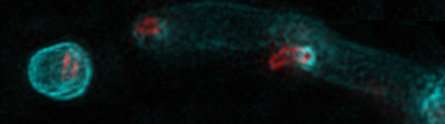
Credit: University of Nottingham
A microbiological mystery of how one bacterium could invade another and grow inside it without breaking the other bacterium instantly has been illuminated by scientists at the University of Nottingham and Indiana University.
The Nottingham scientists are investigating the invasive predatory bacteria Bdellovibrio bacteriovorus as a potential therapeutic to kill antibiotic-resistant pathogenic bacteria. The Indiana scientists are investigating what bacterial cell structures are made of and how they are built. To do this they have developed and used fluorescent D amino-acids (FDAAs) – coloured substitutes for natural substances found in bacterial cell walls. This was combined with super-resolution microscopy to great effect in a new paper published today in Nature Microbiology.
The teams have joined forces and discovered that the invading Bdellovibrio bacterium forms a tiny reinforced molecular 'porthole' in the wall of the host bacterium, squeezes through this and then seals it up from the inside. This process is like cutting and welding a porthole on a ship but on a molecular-scale.
Professor Liz Sockett from the University of Nottingham said: "The bacteria being invaded are 100 million times shorter than a ship like the Queen Mary 2, and the invading bacteria are 500 million times narrower. The materials used for the welding aren't metal of course, but are natural D-amino-acids. These are mirror image forms of the 'L' amino-acids found in the proteins of foods and of our bodies.
"We discovered a second process where the invading bacteria effectively 'plaster' the inside of the bacterium they are invading, again using the D amino-acids. This makes the inside of the bacterium a more reinforced home for the Bdellovibrio to live inside. This is important as a previous paper showed that the invaded bacterial walls are initially rounded-up and weakened early in the invasion process."
Erkin Kuru, a PhD student at the time, suggested to Liz during a lecture visit to Indiana, that she use coloured FDAAs to label the two different bacteria as the predators attacked. Adding a new colour just as invasion was beginning and later as it progressed, replaced the natural amino-acids being used and shone a new coloured light on how predation works.
FDAAs showed what was happening at each stage and gave the team a 'eureka moment' when they saw that the predatory bacteria make a 'porthole' with a central pore surrounded by a reinforcing ring containing D amino-acids. Bdellovibrio squeeze through this pore and fill it in with more D-amino-acid containing material so the invaded bacteria don't burst and all their internal cell contents can be privately eaten by the predators without leaking away to the outside.
As this is happening the predatory bacteria go on to add more FDAAs in all around the wall of the invaded bacterium, not just at the porthole ring. In the experimental conditions the predatory bacteria 'painted' this coloured FDAA, rather like a molecular scale 'fresco', to the walls of the invaded bacterium in a process which reinforces the wall of invaded bacterium so it doesn't collapse before the predator has eaten the nutritional contents inside. Dr Carey Lambert from Nottingham joined the project and was able to find some of the 'tools' that apply the frescos – these are a group of enzymes that have been little studied until recently.
Professor Sockett concludes: "It is remarkable to see this in action at such a tiny scale and also useful. Knowing more about the mechanisms used by the invading predatory bacteria could help design new ways of killing pathogens. Now that the invasion processes have been defined it should be possible to gather all the tools needed to invade and consume pathogenic bacteria without releasing large amounts of their pathogenic cell materials by them bursting."
More information: Erkin Kuru et al. Fluorescent D-amino-acids reveal bi-cellular cell wall modifications important for Bdellovibrio bacteriovorus predation, Nature Microbiology (2017). DOI: 10.1038/s41564-017-0029-y
Journal information: Nature Microbiology
Provided by University of Nottingham