Identifying a new family of light-responsive proteins
When Han Bao started looking for a new cyanobacteria species to study, she had no idea that the perfect candidate was just upstairs.
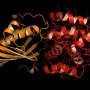
Han is part of the Kerfeld lab's project around the orange carotenoid protein (OCP), a protein that responds to light from the environment to protect its hosts, cyanobacteria (formerly known as "blue-green algae").
The interest in the OCP is two-fold: first, it plays a prominent role in cyanobacterial photosynthesis, and the Kerfeld lab wants to understand how it works.
They then want to use that knowledge to re-engineer this protein for applications in renewable energy and medicine.
And after Han and her labmates conducted a bioinformatical analysis on cyanobacterial genomes (a genome is a complete copy of an organism's DNA blueprint), she found out the Montgomery lab, also at the MSU-DOE Plant Research Laboratory, specializes in a species that would facilitate her study of a new family of OCP proteins she identified.
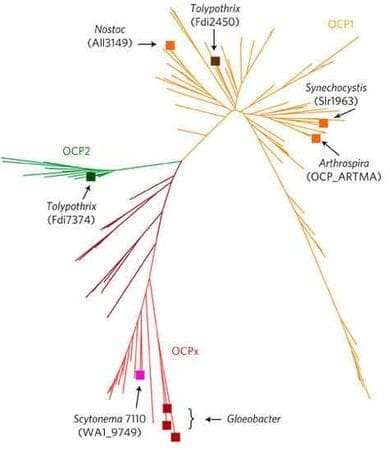
Her study, published in the journal Nature Plants (the article made the journal cover), introduces and describes this new family, called OCP2.
An increasing number of cyanobacteria genomes
"Most of the previous studies on the OCP focus on the one found in a cyanobacterium called Synechocystis," Han says. "This OCP, known as OCP1, is very well studied."
But over the past five years, hundreds of cyanobacterial genomes have become available for analysis.
The data is showing scientists how today's OCPs, and their domain homologs, have evolved over billions of years in different cyanobacteria, gradually diversifying and specializing in different functions.
After all, cyanobacteria are sophisticated organisms, living in dramatically different environments around the planet, from freezing arctic regions to hot springs in Yellowstone National Park.
OCPs have adapted accordingly to protect cyanobacteria from harmful light exposure. And their functional diversity is interesting for developing renewable energy or devising new healthcare tools, which is why the Kerfeld lab want to understand how various OCP families work.
Introducing OCP2
"We did a bioinformatics analysis to analyze all cyanobacteria genomes available in the database," Han says. "We found two new OCP families, beyond the well-studied OCP1. We focused our attention on OCP2, found in the cyanobacterium, Fremyella, which is studied by the Montgomery lab."
Interestingly, OCP evolution has led to both OCP1 and OCP2 being present in Fremyella, creating a great opportunity to compare both families in one organism.
"We found that OCP2 has different properties compared to OCP1. For example, OCP2 reacts much faster to changes in environmental light conditions."
On the other hand, OCP2 needs a comparatively higher light intensity before it is activated to protect the cyanobacterium, while OCP1 can protect it at lower light intensities.
OCP2's structure is also simpler than OCP1's. Han and the Kerfeld lab think such characteristics suggest OCP2 is a more primitive protein.
"We have more evolutionary evidence to back that claim. We know OCP1 has evolved to interact with another protein in Fremyella, called FRP (fluorescence recovery protein). What FRPs do is speed up the OCP1's recovery in the dark. But, OCP2 does not interact with FRP."
Here's what she thinks happened. OCP1 evolved to interact with FRP as a way to add a layer of regulation, in its quest to protect cyanobacteria.
Although that additional interaction slows down OCP1, it makes it better—more refined—or "smarter" at its job.
A good analogy is bureaucratic red tape: the interaction with FRP is like an extra layer of paperwork, which slows down a company's activities. But usually, established bureaucracies are more stable.
But OCP2 being primitive does not mean it is less useful, especially when considering synthetic biology applications.
"Different families have uniquely interesting characteristics. Another study in our lab just showed how OCP1 and OCP2 work differently when we break them apart and look at them. Their different properties will be useful to engineer varying applications, dependent on each family's strengths."
The Kerfeld lab is on the hunt for more OCP families, beyond OCP2, in its continuing quest to build a structural and functional knowledge base about this protein.
More information: Han Bao et al, Additional families of orange carotenoid proteins in the photoprotective system of cyanobacteria, Nature Plants (2017). DOI: 10.1038/nplants.2017.89
Journal information: Nature Plants
Provided by Michigan State University