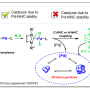
A preparative-scale reaction using platinum clusters with a single-digit atomicity realized
Subnanometer noble-metal clusters have been studied for catalytic applications. For example, platinum is used as a catalyst for fuel cells. Platinum is indispensable as a material for the next-generation energy grid, but it is a rare metal with limited reserves. In order to make effective use of resources, it is essential to improve the atomic-level accuracy of subnanometer metal clusters and increase the amount of synthesis.
While several synthesis methods have been developed from multi-nuclear metal complexes or stable ligand-protected "magic number" clusters, the precursors were not scalable and the exact preservation of atomicity was never provided. This may have been due, in part, to the high metal-to-ligand binding energy that requires an extremely high calcination temperature, which results in the aggregation of the clusters.
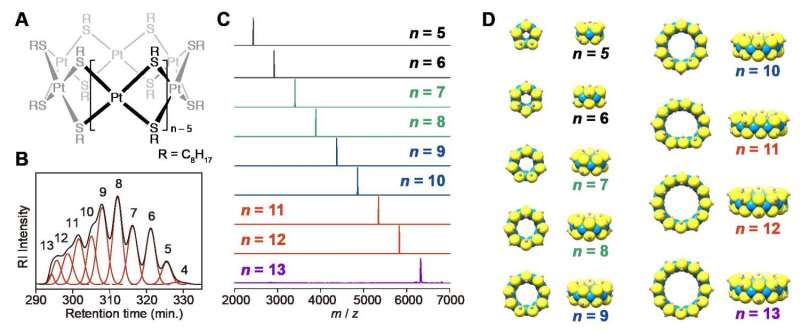
Scientists at Tokyo Tech investigated platinum-thiolates that may lead to the formation of bare metal clusters through reductive metal-sulfur bond cleavage. These platinum-thiolates have been shown to form tiara-like complexes that are stable enough for isolation.
Takane Imaoka, Kimihisa Yamamoto and colleagues studied the monodispersed subnanometer platinum clusters and their catalytic activity for preparative-scale reactions through non-destructive conversion from platinum thiolate complexes to platinum tiara-like clusters. Prior to this study, only one example of a platinum thiolate tiara-like complex with full chemical identification has been published.
As a basis for this study, they began the investigation with basic reactions between PtCl4 and n-octanethiol. Results of these basic reactions suggest that platinum thiolates initially undergo a linear chain growth, followed by entropically favorable formation of smaller cyclic compounds. Based on these results, they optimized the synthesis protocol in two steps resulting in a crude product with platinum tiara-like complexes of various ring sizes.
The platinum tiara-like complexes where further isolated into their pure clusters based on ring sizes through size exclusion chromatography based on preparative recycling HPLC. To affirm the potential of these complexes to be used as precursors for atom-precise platinum clusters, the thermostability and catalytic activity of the complexes were investigated.
The scientists discovered that the tiara-like platinum thiolate complexes were suitable as precursors for the synthesis of monodispersed zero-valent platinum clusters with a specific atomicity—a reaction that previous chemical methods could not realize. This is the first method to demonstrate a preparative-scale reaction using atom precise clusters with a single-digit atomicity, which is expected to be useful in producing high-performance catalysts. Further investigation on the stability and support effect is necessary for the complete understanding of the catalyst system by clusters of such a small size.
More information: Takane Imaoka et al, Platinum clusters with precise numbers of atoms for preparative-scale catalysis, Nature Communications (2017). DOI: 10.1038/s41467-017-00800-4
Journal information: Nature Communications
Provided by Tokyo Institute of Technology