Researchers devise a new way of producing hydrogen fuel

A U.S.-based team of researchers including MIPT scientists has assembled a nanoscale biological structure capable of producing hydrogen from water using light. They inserted a photosensitive protein into nanodiscs—circular fragments of cell membrane composed of a lipid bilayer—and enhanced the resulting structure with particles of titanium dioxide, a photocatalyst. The research findings were published in the journal ACS Nano.
Professor Vladimir Chupin, who heads the Laboratory of the Chemistry and Physics of Lipids at MIPT's Research Center for Molecular Mechanisms of Aging and Age-Related Diseases, says, "Our laboratories working with membrane proteins, in particular with nanodiscs, are mostly focused on biophysical and medical issues. However, the recent joint study with our U.S. colleagues shows that by bringing together biological and technical materials, nanodiscs can be used to obtain hydrogen fuel."
Hydrogen fuel
Hydrogen is one of the cleanest alternative energy sources. When it burns, the only product formed is water vapor. Furthermore, at 45 percent or more, the efficiency of hydrogen fuel is much greater compared with less than 35 percent for gasoline or diesel fuel. Although major automobile manufacturers, such as Toyota, Honda, and BMW, are already producing hydrogen-powered cars, their numbers are modest. Hydrogen is still costly to obtain, largely due to the high power consumption involved. For this reason, researchers are seeking ways of generating this fuel by tapping into other energy sources.
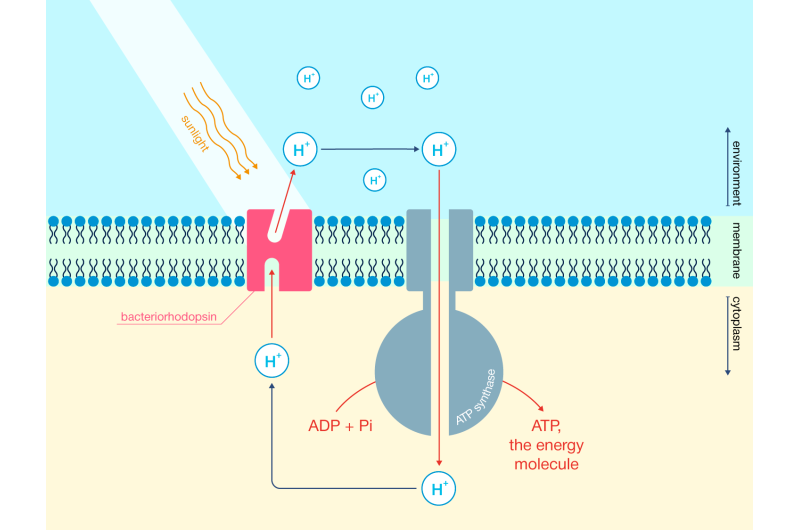
Hydrogen can be produced from water using solar power. The process requires special compounds called photocatalysts. Titanium dioxide is one of the most widely used. It is hardly the most effective photocatalyst, though, so researchers go to great lengths to boost its performance by grinding it to nanosize or adding impurities. To that end, the scientists at Argonne National Laboratory in Illinois, U.S., have turned to biology, assembling a nanostructure made of titanium dioxide and a membrane protein called bacteriorhodopsin. By enhancing each other's performance, these two light-sensitive components form a new system whose capabilities by far exceed those of its constituent parts.
Bacteriorhodopsin is a photosensitive protein that is part of the membranes of some microbial cells. In fact, there are quite a few of such proteins, but the one used in this study was taken from Halobacterium salinarum. One end of the protein sticks out on the outside of a cell, while the other end is on the inside. Sunlight causes bacteriorhodopsin to pump protons out of the cell, which enables the cell to synthesize energy in the form of adenosine triphosphate. Incidentally, the human body produces a total of about 70 kilograms of ATP every day.
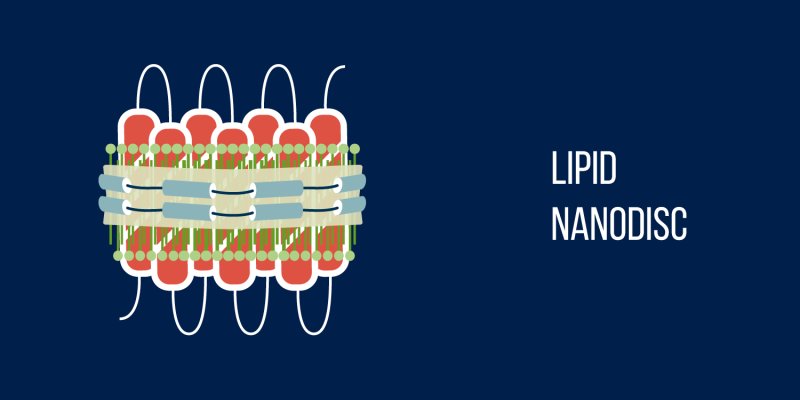
Researchers can now synthesize life artificially, with no biological cells involved. Thus, functional membrane proteins can be obtained by using media that mimic the proteins' natural environment. Among such media available to scientists are nanodiscs—membrane fragments made up of phospholipids and encircled by two protein molecules in a double belt formation. The size of a nanodisc depends on the length of the two belt-like proteins. As a membrane protein, bacteriorhodopsin belongs in a cell membrane and so is quite at home in a nanodisc, which is an amazing structure designed to preserve natural protein structure. Nanodiscs have been used to study membrane protein structures, develop medical agents, and are now being repurposed for photocatalysis. Assisted by MIPT material scientists, the researchers obtained nanodiscs 10 nanometers in diameter, with bacteriorhodopsin nested within.
They did end up with hydrogen
The team dissolved nanodiscs in water, along with titanium dioxide particles. They added platinum, because it makes photocatalysis more effective. Left overnight in that mixture, the nanodiscs stuck to the catalytic particles. Bacteriorhodopsin—the proton pump—doubled up as an antenna. It captured light and transferred its energy to titanium dioxide, increasing its sensitivity to light. In addition, bacteriorhodopsin performed its usual function of translocating protons, which were reduced, yielding hydrogen thanks to the presence of the platinum catalyst. Because it takes electrons to reduce protons, the researchers added some methanol into the solution to serve as an electron donor. The mixture was exposed to green and white light, with some 74 times more hydrogen produced in the latter case. On average, the emission of hydrogen was maintained at a nearly constant rate for at least two to three hours.
Although experiments with a similar nanostructure have been conducted before, they used bacteriorhodopsin in a natural cell membrane. Replacing it with nanodiscs, the researchers produced just as much hydrogen or more, and they even required less bacteriorhodopsin for the same amount of titanium dioxide. The team suspects this could be credited to the capability of the compact and uniform nanodiscs to interface more evenly with the catalytic particles. Although natural bacteriorhodopsin remains the cheaper option, for now, it is possible that the evolution of artificial biosynthesis methods will soon make nanodiscs a more feasible alternative.
More information: Peng Wang et al, Cell-Free Synthetic Biology Chassis for Nanocatalytic Photon-to-Hydrogen Conversion, ACS Nano (2017). DOI: 10.1021/acsnano.7b01142
Journal information: ACS Nano
Provided by Moscow Institute of Physics and Technology