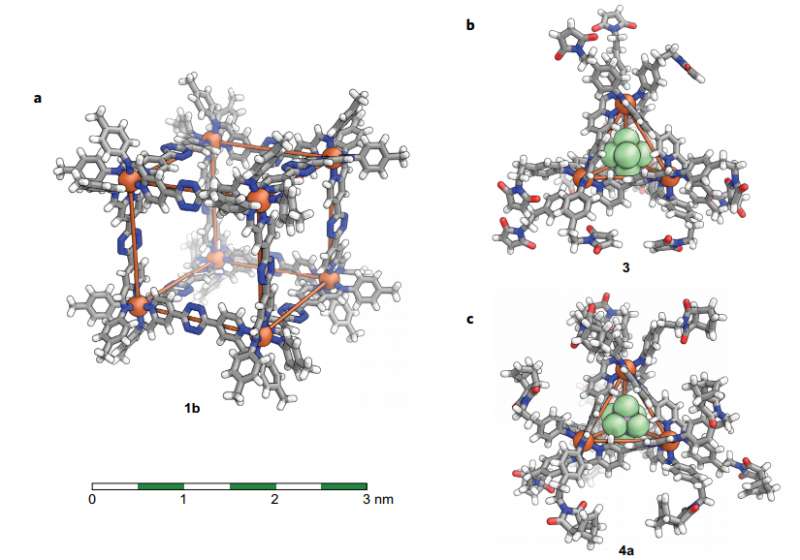
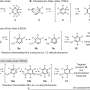
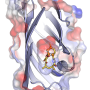
Depictions (same scale) of the X-ray crystal structures. a, Cube 1b. b, PF6 − adduct of tetrahedron 3. c, AsF6 − adduct of modified tetrahedron 4a. Atom colours: grey, C; white, H; red, O; blue, N; orange, Fe; light green, F. Disorder and non-encapsulated anions are omitted for clarity. Iron atoms are connected with orange lines to illustrate the overall geometry of each complex. Credit: (c) Nature Chemistry (2017). DOI: 10.1038/nchem.2839
(Phys.org)—Within the cell chemical messages are passed through a signaling cascade. This cascade can be a series of chemical reactions or molecular changes that spurn the next reaction in a kind of assembly line process. This is how the cell responds to its environment and how communication happens over (biochemically ) long distances.
Inspired by natural signaling cascades, chemists have developed synthetic cascades that modify a self-assembled supramolecular structures post-assembly. Researchers from the University of Cambridge have developed a synthetic cascade that is triggered by norbornadiene (NBD) and relayed by a cyclopentadiene (CPD) intermediate resulting in the triggered change of two supramolecular structures. Their work appears in Nature Chemistry.
After a one-pot self-assembly of two supramolecular structures, the Nitschke group developed a post-assembly modification (PAM) technique via a covalent signal cascade. PAM reactions are limited by the need to be sufficiently mild that they do not damage the supramolecular structure. They also need to be chemoselective and produce product in near-quantitative yields to eliminate the need for extensive purification and isolation. Diels-Alder reactions are good candidates for these types of reactions.
Pilgram, et al. developed a system that underwent inverse-electron demand Diels-Alder (IEDDA) reaction with the initial triggering molecule, NBD, and a tetrazine-edged FeII8L12 cube, and subsequent Diels-Alder reaction with the relay molecule, CPD, and a tetrahedral complex. Their studies verified that the reactions required the triggering molecule and the relay molecule in order to work.
Specifically, the synthetic scheme involved first making the terazine-edged cube in a one-pot synthesis. It then underwent the IEDDA reaction between the tetrazines and the NBD trigger molecule. After conducting model reaction tests, they used 2-octadecylnorbornadiene giving a pyridizine-edged cube and a 1-octadecylcyclopentadiene intermediate. This intermediate served as a relay molecule that bound to the maleimides on the tetrahedral complex via a Diels-Alder reaction.
In one test, the final reaction involved forming a CPD intermediates with sufficiently long alkyl chains that the tetrahedral product transfers from an organic polar phase to a non-polar phase. Notably, without the initial triggering molecule, the final product is not formed and does not move into the non-polar phase, thus demonstrating a synthetic signal cascade in which a molecule changes lipophilicity as a result of an environmental trigger.
Additionally, because the cell's signal transduction pathways are often regulated by both triggering species as well as inhibitors, the Nitschke group investigated potential inhibitor molecules to their synthetic cascade. They found that cyclooctyne served as a good inhibitor by competitively reacting with the terazine molecules over the NBD trigger.
Finally, another key finding of their synthetic cascade is that when the tetrahedral structure is complexed with an anionic guest (PF6-), the guest remains intact even after post-assembly modification of the structure. This allows the guest molecule to be transported from the polar to non-polar phase, which has implications for medicinal and other applications where molecule delivery is hindered by polarity or other environmental features.
Dr. Nitschke told PhysOrg that future research includes designing cascades of reactions that are capable of being pushed backwards under out-of-equilibrium conditions.
According to Nitschke, "Cascades of out-of-equilibrium reactions that are geared together such that one sequence of reactions either up-regulates or down-regulates another are fundamental building blocks of life. Understanding how they might work could help us understand prebiotic chemistry, as well as help us design complex synthetic assembly lines where substrates are passed along between capsules that chemically modify them."
More information: Ben S. Pilgrim et al. Signal transduction in a covalent post-assembly modification cascade, Nature Chemistry (2017). DOI: 10.1038/nchem.2839
Abstract
Natural reaction cascades control the movement of biomolecules between cellular compartments. Inspired by these systems, we report a synthetic reaction cascade employing post-assembly modification reactions to direct the partitioning of supramolecular complexes between phases. The system is composed of a self-assembled tetrazine-edged FeII8L12 cube and a maleimide-functionalized FeII4L6 tetrahedron. Norbornadiene (NBD) functions as the stimulus that triggers the cascade, beginning with the inverse-electron-demand Diels–Alder reaction of NBD with the tetrazine moieties of the cube. This reaction generates cyclopentadiene as a transient by-product, acting as a relay signal that subsequently undergoes a Diels–Alder reaction with the maleimide-functionalized tetrahedron. Cyclooctyne can selectively inhibit the cascade by outcompeting NBD as the initial trigger. Initiating the cascade with 2-octadecyl NBD leads to selective alkylation of the tetrahedron upon cascade completion. The increased lipophilicity of the C18-tagged tetrahedron drives this complex into a non-polar phase, allowing its isolation from the initially inseparable mixture of complexes.
Journal information: Nature Chemistry
© 2017 Phys.org