Used cooking oil can be converted into biofuel with carbon derived from recycled tires -- a new method developed by an Oak Ridge National Laboratory-led research team. Credit: Oak Ridge National Laboratory/Dept. of Energy
Cooking up biofuel
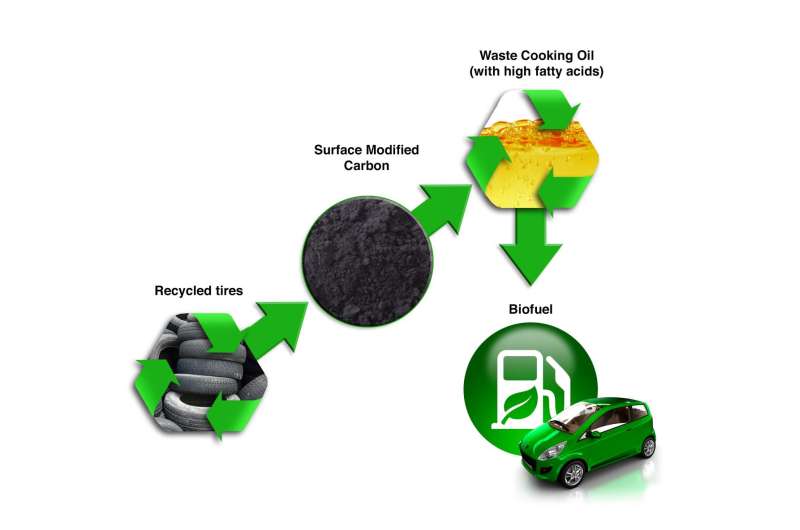
Using a novel, reusable carbon material derived from old rubber tires, an Oak Ridge National Laboratory-led research team has developed a simple method to convert used cooking oil into biofuel. The team's approach combines modified, recovered carbon with sulfuric acids, which is then mixed with free fatty acids in household vegetable oil to produce usable biofuel. The study, done with collaborators Wake Forest University and Georgia Institute of Technology and detailed in Chemistry Select, provides a pathway for inexpensive, environmentally benign and high value-added waste tire-derived products—a step toward large-scale biofuel production, according to ORNL co-author Parans Paranthaman. In previous ORNL studies, carbon powders have proven useful in developing lithium-ion, sodium-ion and potassium-ion batteries and supercapacitors. The patent-pending, waste oil-to-biofuel conversion adds a new approach to waste tire recycling initiatives. [Contact: Sara Shoemaker, (865) 576-9219; shoemakerms@ornl.gov]
Image: https://www.ornl.gov/sites/default/files/news/images/Materials_carbon_to_biofuel_ORNL.jpg
Caption: Used cooking oil can be converted into biofuel with carbon derived from recycled tires—a new method developed by an Oak Ridge National Laboratory-led research team.
Fusion - Blocking the heat
Fusion scientists from Oak Ridge National Laboratory, as part of the DIII-D National Fusion Facility team at General Atomics, are studying an approach to insulate the reactor's innermost wall that surrounds the burning plasma from the energy created when hydrogen isotopes are heated to millions of degrees. The national team created a buffer that traps neutral gas between the plasma's edge, which is cooler than the core but still hotter than the sun, and the interior wall at points where hot ions and atomic particles might make contact. "The trapped, relatively cool particles help maintain the delicate balance of keeping the plasma's core hot enough to produce practical fusion energy and the plasma exhaust cool enough to protect the interior, or first, wall from damaging heat," said ORNL's Aaron Sontag, lead author on a paper published in Nuclear Fusion. "This technique reduces downtime for maintenance and contributes to overarching fusion reactor technology development." [Contact: Sara Shoemaker, (865) 576-9219; shoemakerms@ornl.gov]
Image: https://www.ornl.gov/sites/default/files/news/images/General_Atomics_Tokamak_inside.jpg
Caption: A novel technique can help protect the innermost wall in a fusion reactor from the energy created when hydrogen isotopes are heated to temperatures hotter than the sun. Photo by General Atomics
Chemistry - Discovery doubles output
A simplified catalyst production process developed by Oak Ridge National Laboratory could double the output of high-value chemicals used in making materials found in soda bottles and tires. Scientists found that single gallium cations are the key to increasing production of benzene, toluene and xylenes, or BTX, commodity chemicals commonly used to make plastics and rubber. "Most BTX are produced from fossil fuel, which is energy intensive," said ORNL's Zhenglong Li, co-author of the study published in Green Chemistry. "Our process creates a greener pathway that doubles BTX production from renewable ethanol by introducing gallium into zeolite catalysts." The team's new catalyst production method works without water and reduces costs. [Contact: Kim Askey, (865) 946-1861; askeyka@ornl.gov]
Image: https://www.ornl.gov/sites/default/files/news/images/BTX_story_tip_image.jpg
Caption: Scientists at Oak Ridge National Laboratory created a new catalyst production process that doubles the output of renewable BTX, a group of high-value chemicals used to produce soda bottles and tires.
Batteries - Promising electrode material
An Oak Ridge National Laboratory-led team discovered that vanadium dioxide in a crystalline thin film makes an outstanding electrode for lithium-ion batteries. Theory and computation predicted a high capacity for lithium storage, which experiments confirmed with tests in coin cells. Advanced microscopy proved lithium ions pack into a rigid framework, and ions speed through sites favorable for their adsorption which are abundant along the open channels. Because the material is difficult to grow, it had never been tested. ORNL's Ho Nyung Lee and his team used an advanced synthesis technique to fabricate thin-film crystals and demonstrated that they remained stable even after numerous electrochemical charge/discharge cycles. "The research provides a design strategy for more efficient, long-lived, miniaturized ionic conductors," said Panchapakesan Ganesh of ORNL, who predicted vanadium dioxide's theoretical capacity and lithium ion pathways. "We're developing novel materials and architectures to provide energy solutions for future technologies," Lee said. [Contact: Dawn Levy, (865) 576-6448; levyd@ornl.gov]
Image: https://www.ornl.gov/sites/default/files/news/images/Batteries_promising_electrode_mats_ORNL.jpg
Caption: Researchers predicted where lithium ions (green spheres) would pack and move in an open framework of epitaxially strained vanadium dioxide, depicted here by a stick model (oxygen-connecting bonds are red and vanadium-connecting bonds, turquoise). Guided by theory and computation, they designed, synthesized and tested the material—proving it indeed had excellent storage capacity, ion conduction and structural stability. Image by Panchapakesan Ganesh, Oak Ridge National Laboratory/Dept. of Energy
More information: Zachary D. Hood et al, Novel Acid Catalysts from Waste-Tire-Derived Carbon: Application in Waste-to-Biofuel Conversion, ChemistrySelect (2017). DOI: 10.1002/slct.201700869
Journal information: Green Chemistry
Provided by Oak Ridge National Laboratory