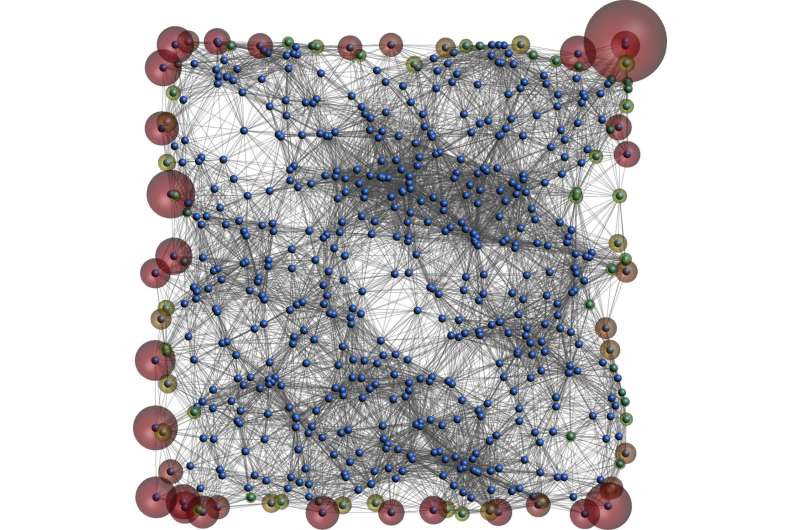
Image shows an electronic state residing on the edge of the amorphous system Credit: Adhip Agarwala and Vijay Shenoy
For the last decade, scientists have sought topological insulators, materials that are insulating on the inside but conduct current on their surfaces. Although first predicted around 2005, very few real-world examples have been found to date. Topological insulators are expected to have wide-ranging applications, including energy-efficient electronics and quantum computing—their special properties allow the surface current to flow freely even in the presence of defects or disturbances.
Until now, scientists have looked for topological insulators among crystals or other materials whose atoms are arranged in a regular fashion. A new study, however, predicts that topological insulators can also be found among amorphous materials, such as some forms of glass, in which atoms are randomly arranged.
The prediction, based on computer models, opens up new avenues in the quest for these materials. "Now there are many more opportunities to find topological insulators," says senior author Vijay Shenoy of the Indian Institute of Science (IISc). Amorphous topological insulators may also be easier to make than crystalline ones, which require stringent controls, he suggests. Shenoy and graduate student Adhip Agarwala carried out the study published in Physical Review Letters.
Topological insulators owe their superior abilities to the presence of special energy states on their surfaces. For current to flow in a material, electrons need to jump from the valence band energy state to a higher state called the conduction band. If the gap between the bands is very large, as found in normal insulators, electrons cannot jump and current does not flow. On the inside, topological insulators have a large band gap and do not conduct current. On their surface, however, electrons occupy certain "mid-gap" states between the valence and conduction bands, which allows them to carry current.
When these materials were first predicted, the theory was based on the assumption that the material structure must be crystalline, says Shenoy. "After some poking around, we found that this is not a crucial assumption. It is not a necessary condition for you to obtain a topological phase," he says.
Shenoy and Agarwala used computer models to "construct" 2-D and 3-D structures in which sites are arranged randomly and electrons can hop between them. Then they tweaked certain parameters such as distance between sites and spacing between energy bands. Under certain conditions, they found that the materials showed mid-gap states on the surface and other mathematical signatures found in topological insulators, despite their random structure.
"People have been only looking at crystalline materials. And they haven't found very good topological insulators," says Agarwala. "Even theoretically, people can now look at many, many substances, not only amorphous materials. We have shown for the 'worst-case scenario' where the structure is completely random. We can think about many more materials in between crystalline and amorphous, and ask if topological insulators can exist."
Researchers could also look at other ways to make topological insulators, the authors suggest. One possibility, for example, is to randomly add atoms with appropriate energy levels to the surface of an existing insulator to give rise to topological states.
Topological insulators have special properties that make them attractive for electronics. For example, the direction in which the surface electrons spin is locked to the direction in which they move. This locking prevents defects or impurities from changing the electron's spin and therefore knocking it off its path, thus minimizing current loss.
"One of the active areas in condensed matter physics and materials science is to find such materials," says Shenoy. "If found, it will be an important discovery and could drive the next round of electronics."
More information: Topological Insulators in Amorphous Systems, published in Physical Review Letters, June 2017. doi.org/10.1103/PhysRevLett.118.236402
Journal information: Physical Review Letters
Provided by Indian Institute of Science