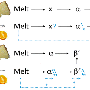When life gives you lemons, make bioplastics

From your phone case to airplane windows, polycarbonates are everywhere. Several million tons of polycarbonate are produced every year around the world. However, worries about the dangers of this material are increasing because of the toxicity of its precursors, especially bisphenol-A, a potential carcinogen.
Now, a team of chemists led by Arjan Kleij, ICIQ group leader and ICREA professor, have developed a method to produce polycarbonates from limonene and CO2, both abundant natural products. Limonene can replace a dangerous compound currently used in commercial polycarbonates—bisphenol-A (BPA). Although BPA has been repeatedly classified as a safe chemical by American and European agencies, some studies conclude that it is a potential endocrine disruptor, neurotoxin, and carcinogen. Some countries, including France, Denmark and Turkey, have banned the use of BPA in the production of baby bottles.
'BPA is safe, but still causes concerns and is produced from petroleum feedstock,' Kleij points out. 'Our approach replaces it with limonene, which can be isolated from lemons and oranges, giving us a much greener, more sustainable alternative,' he says. Because fully replacing BPA for limonene is complicated, Kleij explains that BPA is difficult to replace. 'We can start adding small quantities of limonene, then progressively substituting BPA,' he says. 'Step by step, the adaptation process could lead to new limonene-derived biomaterials with similar, or even enhanced and novel properties.'

The researchers not only succeeded in producing a more environmentally friendly polymer, they also managed to improve its thermal properties. This limonene-derived polymer has the highest glass transition temperature ever reported for a polycarbonate. 'We were quite surprised to find this, because known bio-plastics have worse thermal properties than classic polymers,' explains Kleij. 'We were at first sceptical about these findings, but we were able to reproduce these features consistently.' Having a high glass transition temperature has other implications. The new plastics require higher temperatures to melt, which make them safer for everyday use. Moreover, this new polymer also offers many new applications for polycarbonates and block copolymers using appropriate material formulations.
Kleij and co-workers are currently negotiating with plastic producers to further advance the industrial manufacture of limonene-derived biomaterials.
More information: Nicole Kindermann et al, Access to Biorenewable Polycarbonates with Unusual Glass-Transition Temperature (Tg) Modulation, ACS Catalysis (2017). DOI: 10.1021/acscatal.7b00770
Journal information: ACS Catalysis
Provided by Institute of Chemical Research of Catalonia (ICIQ)