Breakthrough achieved in improving the ionic conductivity of fuel cell materials
Ceramic fuel cell technology has a tremendous potential for clean energy production.
The researchers at Aalto University developed synthesis and processing routes for development of ceramic nanocomposite materials, which resulted in a breakthrough in improving the ionic conductivity of the fuel cell electrolyte materials.
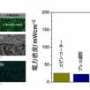
A record high ionic conductivity of 0.55 S/cm at 550oC has been achieved at Aalto University. Fuel cells fabricated using these nanocomposite materials produced an outstanding performance of 1.06 W/cm2.
Ceramic fuel cell technology has a tremendous potential for sustainable clean energy production. With the help of these superionic nanocomposite materials, the operating temperature of the fuel cells can be significantly reduced. This low temperature operation helps in improving the long-term stability of the devices.
'With the help of these superionic materials, the losses due to ionic transport in the electrolyte layer are dramatically reduced, which makes it possible to produce fuel cells performing over 1W/cm2. We envision to reach a fuel cell performance of 2.5 W/cm2 by depositing these potential materials with modern printing method', Docent, Dr. Muhammad Imran Asghar says.
This work is a part of an EU-Indigo project funded by the Academy of Finland. The partners in the project include Aalto University, University of Oslo, University of Aveiro, Indian Institute of Technology – Delhi, CGRI – CSIR Kolkata and VESTEL Turkey.
The synthesized superionic materials were characterized with various microscopic (SEM, TEM), spectroscopic techniques (XRD, Raman, FTIR) and other analyses (BET analysis, DSC, TGA) techniques. The high performance fuel cells were characterized using electrochemical impedance spectroscopy and voltage/current-density measurements.
Details regarding the results can be found in the articles published in International Journal of Hydrogen Energy and Frontiers of Chemical Science and Engineering.
More information: Muhammad I. Asghar et al. Comparative analysis of ceramic-carbonate nanocomposite fuel cells using composite GDC/NLC electrolyte with different perovskite structured cathode materials, Frontiers of Chemical Science and Engineering (2017). DOI: 10.1007/s11705-017-1642-2
Ieeba Khan et al. High conductive (LiNaK) 2 CO 3 Ce 0.85 Sm 0.15 O 2 electrolyte compositions for IT-SOFC applications, International Journal of Hydrogen Energy (2017). DOI: 10.1016/j.ijhydene.2017.05.152
Journal information: International Journal of Hydrogen Energy
Provided by Aalto University