From battery waste to electrochemical sensor
Multiplex detection of antioxidants / food additives / preservatives in food samples is possible using our newly developed graphite-based nanocomposite electrochemical sensor from used alkaline battery. The chemical sensor not only leads to shorter analysis time but also is a greener chemistry innovation.
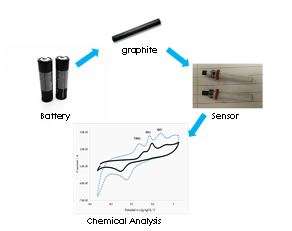
A small AA battery can do the important job of powering up our remote control, mini toys and alarm clock, but after reaching its life spent, there is environmental issue that we need to solve. A typical zinc battery is composed of a zinc body, manganese powder, paper, starch and a black rod that make the battery works. Most of the parts are recyclable, but not the black rod (which scientists referred as the "graphite rod"). This material possesses good electrical conductivity property and can actually be reused for the development of a chemical sensor.
The graphite rod that was extracted out from the used battery can be cut into various shapes, either in rods, buttons, or thin sheets. Besides, it can also be fabricated into small chip devices and attached onto human skin or as a strip for the detection of chemical substances in food. Food additives (chemicals) such as anti-oxidants or preservatives could be piece of interesting information, whereby most people are concern of and would like to know their actual amount before consuming.
The possibility of miniaturizing a laboratory into graphite chip or strip to give us the instant information regarding the dosage intake of anti-oxidants or preservatives in our daily meals is achievable through a simple and economical graphite rod conversion steps. In contrast to the conventional laboratory tests that could take up a day for chemical analysis, a portable, affordable and accurate small analytical device is more preferred. The development of chemical sensors has begun a decade ago, as its potential use is promising due to high demand. Alas, the cost for such development using expensive sensor materials is not affordable.
To overcome this challenge, our research group has found an exciting solution by looking into reused battery waste. We have successfully fabricated a number of graphite nanocomposite electrochemical sensors by surface modification with nanomaterials, which significantly improved the materials' chemical and physical properties that fit to its usage as a chemical sensor. We have demonstrated the practical use of the developed graphite-based electrochemical sensor for the quantitative detection of Myricetin (natural anti-oxidant) and multiplex detection of other preservatives (synthetic organic molecules) in different forms of actual food samples. The analysis results obtained was found well correlated to the conventional laboratory test results using HPLC. More importantly, the test conducted using our developed sensor method is relatively faster whereby results could be read in less than 5 minutes. In addition, the recycled graphite rod used is an inert material. Hence, it is safe to be used and will not cause any harmful effect to the end users. This is another added value to the newly developed alternative analytical approach.
To conclude, the innovative attempt of developing a chemical sensor from battery waste not only brings benefits to the end users, but also is a caring effort shown in practicing green chemistry for a greener earth and better tomorrow.
More information: Khan Loon Ng et al. Graphite nanocomposites sensor for multiplex detection of antioxidants in food, Food Chemistry (2017). DOI: 10.1016/j.foodchem.2017.06.029
Journal information: Food Chemistry
Provided by University of Malaya