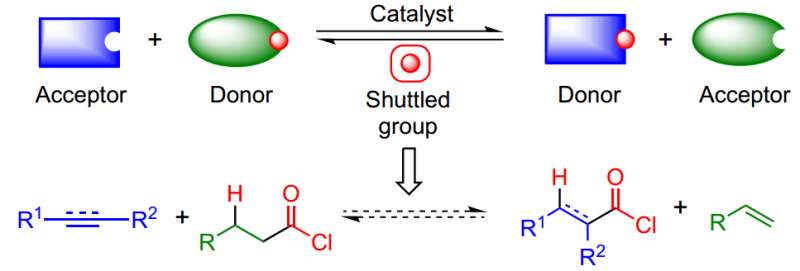
The approach takes advantage of an isodesmic shuttle-catalysis process to elude the need for CO and HCl and overcome the thermodynamic challenge of making highly reactive products. Credit: Nature Chemistry (2017). DOI: 10.1038/nchem.2798
(Phys.org)—The synthesis of carboxylic acid derivatives from unsaturated carbon compounds is important for making chemicals used in pharmaceuticals, cosmetics, polymers, and agrochemicals. In industry this reaction is done using high pressure carbon monoxide along with the appropriate catalyst for the job. While this may be possible in certain facilities, the use of a poisonous gas is not feasible in the laboratory setting. Additionally, because these reactions often require a tailor-made catalyst, the process is limited to only those substrates that work with the catalyst.
Researchers at the Max Planck Institute have developed a synthesis that is much broader in scope and avoids the use of high pressure CO and corrosive HCl gas. Xianjie Fang, Bastien Cacherat, and Bill Morandi developed a new one-pot synthesis that yields a variety of carboxylic acids as well as amides and thioesters. Their synthesis employs a catalytic shuttle that donates the CO and HCl needed to produce an acid chloride intermediate that is then converted to the desired product. Their work appears in Nature Chemistry.
Shuttle catalysis eliminates the need to use dangerous reagents and opens the door to using nucleophiles that were not accessible under other reaction conditions (i.e., Reppe carbonylation) According to Dr. Morandi, "The catalyst effectively acts as a shuttle for the transport of a chemical group between one molecule (donor) and another molecule (acceptor) in a reversible manner."
In this paper, the authors combined an acid chloride donor and an unsaturated substrate to produce the desired acid chloride that can then be converted to a wide range of useful functional groups. Their shuttle was Pd/xantphos, a catalytic system that has been used in other types of alkene reactions.
The key to their synthesis was to find an appropriate CO and HCl donor molecule. As a model reaction, Fang et al. used cyclododecyne, an internal alkyne, and tried various low-molecular weight aliphatic acid chlorides as their donor molecules. They determined that butyryl chloride was their best candidate because it is inexpensive, has a low molecular weight, and has good reactivity.
They then investigated the scope of their reaction mechanism. Several of the internal alkynes that they investigated only required a little more than one equivalent of butyryl chloride and produced the desired acid chloride product in good yields. Furthermore, because the reaction is sensitive to steric effects, the products showed an excess of one regio product and selectively reacted at the distal position in cases where there was more than one option for reaction.
The reaction conditions also worked for terminal alkynes. For this reaction, about four equivalents of butyryl chloride were required and the branched isomer was isolated from the reaction to give the desired product in good yield. The authors noted that they were able to isolate alkyl acrylate products, which are important compounds for polymer chemistry. They alos tested their reaction with halogens, protected alcohols, esters, a ketone, a nitrile, and phthalimides, all of which were well tolerated in this reaction.
Fang et al. also tested how this reaction fared with alkenes. They produced bicycle acid chlorides from strained alkenes in good yields. Terminal alkenes gave the corresponding acid chloride in adequate yields.
The next step in their procedure was to do a one-pot synthesis by converting the acid chloride to the corresponding carboxylic acid, thioester, or amide. They began by looking at nucleophiles that are typically inaccessible when done with previously reported reactions. These included a bulkyl tertiary alcohol and tocopherol. They also tested indole and a tertiary thiol. They also were able to conjugate cinchonine and estrone, two bioactive molecules.
Finally, their one-pot synthesis was tested to see if it could be used to make other carbonyl compounds. These required using different initial substrates and a second catalyst. These included Friedel-Crafts reactions as well as other coupling reactions to make ketones.
In regards to the broader implications of their work, Dr. Morandi said that "this work demonstrates that shuttle catalysis is not only a valuable approach to avoid the use of toxic gases, but also a powerful tool to discover novel reactivity that is challenging to obtain otherwise"
More information: Xianjie Fang et al. CO- and HCl-free synthesis of acid chlorides from unsaturated hydrocarbons via shuttle catalysis, Nature Chemistry (2017). DOI: 10.1038/nchem.2798
Abstract
The synthesis of carboxylic acid derivatives from unsaturated hydrocarbons is an important process for the preparation of polymers, pharmaceuticals, cosmetics and agrochemicals. Despite its industrial relevance, the traditional Reppe-type carbonylation reaction using pressurized CO is of limited applicability to laboratory-scale synthesis because of: (1) the safety hazards associated with the use of CO, (2) the need for special equipment to handle pressurized gas, (3) the low reactivity of several relevant nucleophiles and (4) the necessity to employ different, often tailor-made, catalytic systems for each nucleophile. Herein we demonstrate that a shuttle-catalysis approach enables a CO- and HCl-free transfer process between an inexpensive reagent, butyryl chloride, and a wide range of unsaturated substrates to access the corresponding acid chlorides in good yields. This new transformation provides access to a broad range of carbonyl-containing products through the in situ transformation of the reactive acid chloride intermediate. In a broader context, this work demonstrates that isodesmic shuttle-catalysis reactions can unlock elusive catalytic reactions.
Journal information: Nature Chemistry
© 2017 Phys.org