Female babies are born with a full set of egg precursors in their ovaries, yet the molecular mechanism by which these cells proliferate during embryonic development was unclear. Now, using a mouse model created at A*STAR, an international team of researchers has pinpointed the regulatory factors needed for this rapid cell division to occur in the developing female gonad.
"We have paved the way to study different cell cycle regulatory pathways that may go awry during development," says study author Philipp Kaldis, a senior principal investigator at the A*STAR Institute of Molecular and Cell Biology. Future research in this area, he notes, could lead to new treatments for cancer and infertility.
The embryonic cells that give rise to eggs are known as primordial germ cells, or PGCs. In mice—which have a similar but faster gestation than humans—PGCs are identifiable at around the 7th day of development. By day 8, these cells temporarily stop dividing as they migrate inside the embryo. Then, around day 9.5, the cells enter a three-day period of frenetic growth in which they duplicate every 12 hours and the total number of PGCs increases around 50-fold.
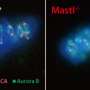
Kaldis suspected that a protein called MASTL might be involved in this 72-hour bonanza of cell division since he and others had previously shown that MASTL is essential for the cell cycle to move forward in other cell types and other species.
He thus genetically engineered a mouse in which he could selectively delete the gene encoding MASTL from PGCs. Kaldis then sent the mice to Kiu Liu and Sanjiv Risal at the University of Gothenberg in Sweden, and collectively they showed that the PGCs in these mice could not complete the anaphase step in the cell cycle, in which the duplicated sets of chromosomes are meant to separate inside the dividing cell.
As a result, the PGCs were defective and died instead of multiplying. However, Kaldis and his team showed that proper cell division could be restored in the MASTL-deficient mice if they simultaneously wiped out another cell cycle regulator called PP2A.
The researchers concluded that MASTL normally functions to suppress the activity of PP2A to enable anaphase to proceed properly. And since defects in these germ cells often lead to tumors or infertility, it's possible, Kaldis notes, that MASTL and PP2A are implicated in these health problems as well. "We hope this work will stimulate new research in PGCs," he says.
More information: Sanjiv Risal et al. MASTL is essential for anaphase entry of proliferating primordial germ cells and establishment of female germ cells in mice, Cell Discovery (2017). DOI: 10.1038/celldisc.2016.52