Building a biological control switch with light, genetics, and engineering ingenuity

A user-friendly switch for controlling room temperature, the thermostat is a classic example of the kind of tools engineers build.
For biological systems research, Megan McClean and Cameron Stewart have taken the idea of a thermostat several steps further—and using their invention, which combines the power of light, computers and genetics, researchers can now build an "optostat" that is remarkably similar to the thermostat in our homes.
"All you need is three ingredients," says McClean, an assistant professor of biomedical engineering at the University of Wisconsin-Madison. "An organism that grows well in cell culture, the ability to insert a light-sensitive switch into its genome, and a computer-controlled microscope that images what you want the organism to produce."
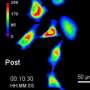
The optostat is a fully automated system that connects these three ingredients with electronics and freely available software. Using the light-responsive part of a plant protein to control the expression of a single gene in baker's yeast, the researchers were able to record images of the fluorescent protein produced by that gene continuously for up to 10 days, capturing how the cells responded to the amount of light they received. While light controlled the expression of the gene of interest, it did not affect the transcription of thousands of other yeast genes.
The system contains everything the cells need to grow in excess, except for one limiting nutrient that is provided through controlled release. Like a thermostat, the optostat can automatically adjust the amount of light needed to obtain a desired protein concentration.
Stewart and McClean recently described their optogenetic system in the Journal of Visualized Experiments, allowing other researchers—especially biologists without an engineering background—to set it up in their own labs.

Stewart compares their invention to a car's cruise control system. "Cars, throttles, and speedometers already existed, but cruise control combined them with a feedback system," he explains. "In our case, growing cells in a 'chemostat' to maintain a constant growth rate has been possible since the 1950s. But our novel contribution is to connect this chemostat to a light bulb to administer inputs, and to a microscope to measure outputs."
The new optostat is the only system of its kind that can sample and monitor the same cell culture continuously over a long period of time. This allows researchers to study any biological pathway of interest by tuning a single parameter and keeping everything else, including the cells' growth rate, the same.
Optogenetics—the use of light-sensitive proteins as regulators of a variety of cellular processes—has been a growing research field for the last ten years, McClean says. Since the response of plants to light has been studied extensively, plant-derived proteins make ideal optogenetic tools.
One application of optogenetic systems that McClean is particularly interested in involves Candida albicans, the most common of more than 20 species of yeast-like fungi that live in our intestinal tract. They are usually harmless, but their overgrowth can trigger infections in certain body parts, such as the mouth or throat (thrust) and the vagina (yeast infection). When the fungus enters the bloodstream and spreads through the body, it may cause dangerous invasive infections.
Some Candida species have recently caused severe illness in hospitalized patients and are now considered a global health threat. "Our drug arsenal for fungi is very limited because these organisms are so similar to our own cells," McClean says. "That makes their emerging resistance to antifungal drugs especially disconcerting."
Candida species are a threat to hospitalized patients because they tend to form a thin mat, or biofilm, on hip or knee implants and intravenous catheters. By controlling different regulators of C. albicans growth with a light-sensitive switch, McClean hopes to learn what makes the organism change from its stable form in a biofilm—long and skinny—to its less stable, round form that may pop off the biofilm and disperse into the bloodstream. In the future, that knowledge may help inhibit fungal infections in humans without causing toxic side effects.
"One of the unique aspects of fungal biology is its potential to disperse into the bloodstream," McClean says. "In order to study the factors that cause it, we need a controllable system that allows time for a biofilm to form and then make light-induced perturbations. With several modifications we plan to implement next, we believe our optogenetic system will eventually provide that kind of tool."
Journal information: Journal of Visualized Experiments
Provided by University of Wisconsin-Madison