Researchers discover new antibiotic effective against drug-resistant bacteria
Scientists from Rutgers University-New Brunswick, the biotechnology company NAICONS Srl., and elsewhere have discovered a new antibiotic effective against drug-resistant bacteria: pseudouridimycin. The new antibiotic is produced by a microbe found in a soil sample collected in Italy and was discovered by screening microbes from soil samples. The new antibiotic kills a broad spectrum of drug-sensitive and drug-resistant bacteria in a test tube and cures bacterial infections in mice.
In a paper published in Cell today, the researchers report the discovery and the new antibiotic's mechanism of action.
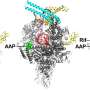
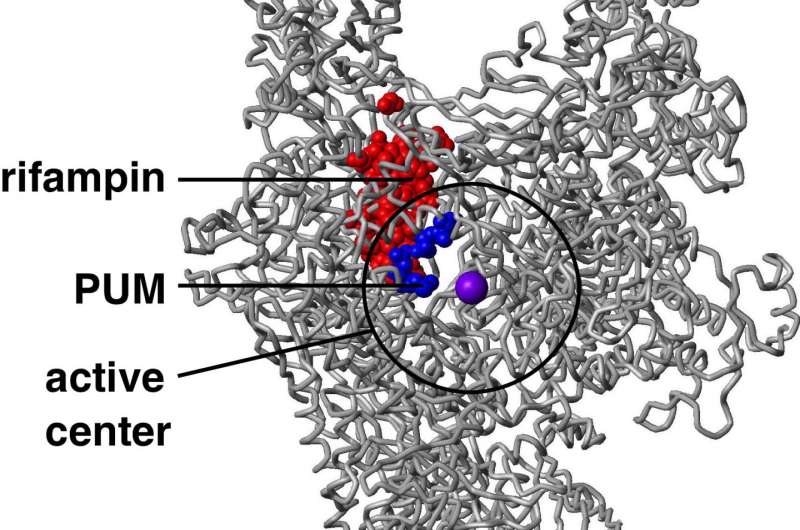
Pseudouridimycin inhibits bacterial RNA polymerase, the enzyme responsible for bacterial RNA synthesis, through a binding site and mechanism that differ from those of rifampin, a currently used antibacterial drug that inhibits the enzyme. Because pseudouridimycin inhibits through a different binding site and mechanism than rifampin, pseudouridimycin exhibits no cross-resistance with rifampin, functions additively when co-administered by rifampin and, most important, has a spontaneous resistance rate that is just one-tenth the spontaneous resistance rate of rifampin.
Pseudouridimycin functions as a nucleoside-analog inhibitor of bacterial RNA polymerase, meaning that it mimics a nucleoside-triphosphate (NTP), the chemical "building block" that bacterial RNA polymerase uses to synthesize RNA. The new antibiotic binds tightly to the NTP binding site on bacterial RNA polymerase and, by occupying the NTP binding site, prevents NTPs from binding.
Pseudouridimycin is the first nucleoside-analog inhibitor that selectively inhibits bacterial RNA polymerase but not human RNA polymerases.
"Because the NTP binding site of bacterial RNA polymerase has almost exactly the same structure and sequence as the NTP binding sites of human RNA polymerases, most researchers thought it would be impossible for a nucleoside-analog inhibitor to inhibit bacterial RNA polymerase but not human RNA polymerases," said Richard H. Ebright, Board of Governors professor of chemistry and chemical biology and laboratory director at the Waksman Institute of Microbiology at Rutgers-New Brunswick, who led the research.
"But pseudouridimycin contains a side-chain that 'reaches' outside the NTP binding site and 'touches' an adjacent site that is present in bacterial RNA polymerase but not in human RNA polymerases and, as a result, it binds more tightly to bacterial RNA polymerase than to human RNA polymerases," Ebright said.
The fact that pseudouridimycin functions as a nucleoside-analog inhibitor explains the low rate of emergence of resistance to the compound.
"The new antibiotic interacts with essential residues of the NTP binding site that cannot be altered without losing RNA polymerase activity and bacterial viability," Ebright said. "Alterations of the NTP binding site that disrupt binding of the new antibiotic also disrupt RNA polymerase activity, resulting in dead bacteria, rather than resistant bacteria."
"Nucleoside-analog inhibitors that selectively inhibit viral nucleotide polymerases have had transformative impact on the treatment of HIV-AIDS and hepatitis C," said Stefano Donadio, CEO of NAICONS Srl., who co-led the research. "The anti-AIDS drugs Zidovudine, Videx, Zalcitabine, Lamivudine, and Viread are nucleoside-analog inhibitors, and the anti hepatitis-C drugs Solvadi and Harvoni are nucleoside-analog inhibitors."
"Nucleoside-analog inhibitors that selectively inhibit bacterial RNA polymerase could have a similarly transformative impact on the treatment of bacterial infections," Donadio said.
"The discovery also underscores the importance of natural products in providing new antibiotics," he said. "Microbes have had had billions of years to develop 'chemical weapons' to kill other microbes."
More information: Cell (2017). DOI: 10.1016/j.cell.2017.05.042
Journal information: Cell
Provided by Rutgers University