Ammonia on-demand? Alternative production method for a sustainable future
Our society is in need of ammonia more than ever. Chemical fertilizers, plastic, fibers, pharmaceuticals, refrigerants in heat pumps, and even explosives all use ammonia as raw material. Moreover, ammonia has been suggested as a hydrogen carrier recently because of its high hydrogen content.
In the Haber-Bosch process, which is the main method of ammonia synthesis, nitrogen reacts with hydrogen using a metal catalyst to produce ammonia. However, this industrial process is conducted at 200 atm and high reaction temperatures of nearly 500°C. Additionally, ammonia production requires using much natural gas, so scientists have been looking for alternative methods to sustainably synthesize ammonia at low temperature.
In a recent study, researchers from Waseda University and Nippon Shokubai Co. Ltd. achieved a highly efficient ammonia synthesis at low temperature, with the highest yield ever reported.
"By applying an electric field to the catalyst used in our experiment, we accomplished an efficient, small-scale process for ammonia synthesis under very mild conditions," says Professor Yasushi Sekine of Waseda University. "Using this new method, we can collect highly pure ammonia as compressed liquid and open doors to developing on-demand ammonia production plants that run on renewable energy."
This research was published in Chemical Science.
In 1972, ruthernium (Ru) catalyst with alkali metals was found to decrease the reaction temperatures and pressures necessary for Haber-Bosch processing, and different methods have been suggested since this discovery. Unfortunately, the ammonia synthesis rate was hindered by kinetic limitations.
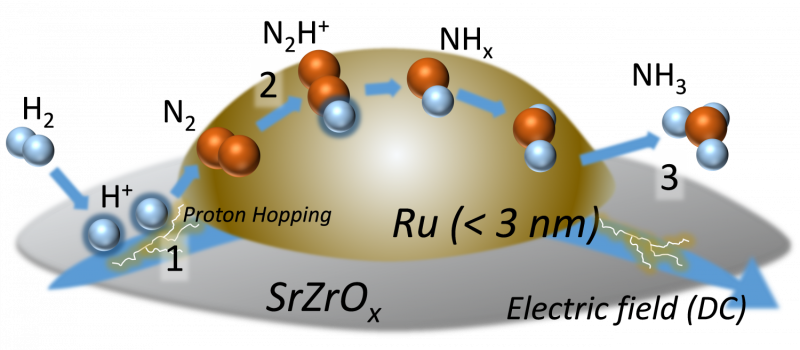
"We applied direct current electric field to the Ru-CS catalyst for our ammonia synthesis. Our research group obtained remarkably high ammonia field of approximately 30 mmol gcat-1h-1 with high production energy efficiency. Not to mention, this was done at low reaction temperatures and pressures from atmospheric to 9 atm, which is kinetically controllable. The energy consumption to produce ammonia was very low as well."
How the researchers were able to obtain such results could be explained by a mechanism called surface proton hopping, a unique surface conduction triggered by an electric field.
"Our experimental investigations, including electron microscope observation, infrared spectroscopy measurements, and isotopic exchange tests using nitrogen gas, prove that proton hopping plays an important role in the reaction, as it activates nitrogen gas even at low temperatures and moderates the harsh condition requirements," explains Professor Sekine.
The new technique also addresses obstacles in conventional ammonia synthesis, such as hydrogen poisoning of Ru catalysts and delay in nitrogen dissociation. Furthermore, the research results suggest that smaller-scale, more dispersed ammonia production could be realized, and building highly-efficient ammonia plants that run on renewable energy will become possible. Such ammonia plants are expected to produce 10 to 100 tons of ammonia per day. Professor Sekine believes that their findings will be important for future energy and material sources.
More information: R. Manabe et al, Electrocatalytic synthesis of ammonia by surface proton hopping, Chem. Sci. (2017). DOI: 10.1039/c7sc00840f
Journal information: Chemical Science
Provided by Waseda University