Total synthesis of flueggenine C via an accelerated intermolecular Rauhut-Currier reaction
The first total synthesis of dimeric securinega alkaloid (-)-flueggenine C was completed via an accelerated intermolecular Rauhut-Currier (RC) reaction. The research team led by Professor Sunkyu Han in the Department of Chemistry succeeded in synthesizing the natural product by reinventing the conventional RC reaction.
The total synthesis of natural products refers to the process of synthesizing secondary metabolites isolated from living organisms in the laboratory through a series of chemical reactions. Each stage of chemical reaction needs to be successful to produce the final target molecule, and thus the process requires high levels of patience and creativity. For that reason, the researchers working on natural products total synthesis are often called "molecular artists".
Despite numerous reports on the total synthesis of monomeric securinegas, the synthesis of dimeric securinegas, whose monomeric units are connected by a putative enzymatic RC reaction, has not been reported to date.
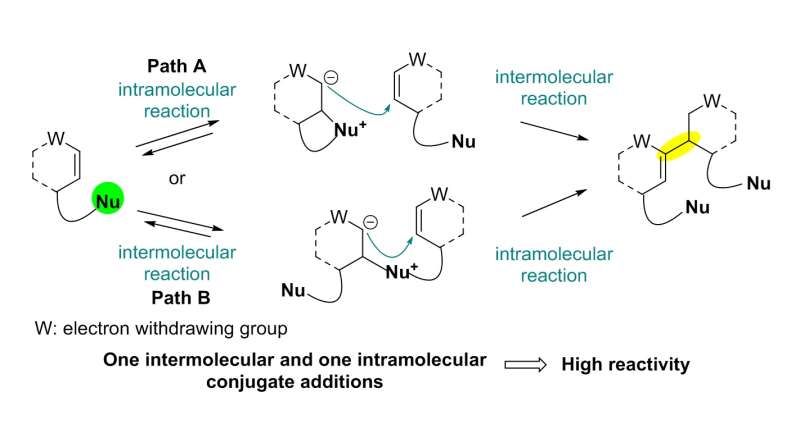
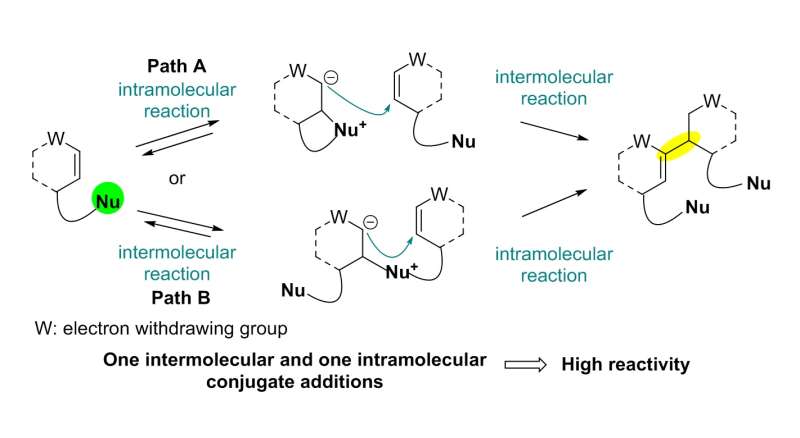
The team used a Rauhut-Currier (RC) reaction, a carbon-carbon bond forming a reaction between two Michael acceptors first reported by Rauhut and Currier in 1963, to successfully synthesize a dimeric natural product, flueggenine C. This new work featured the first application of an intermolecular RC reaction in total synthesis.
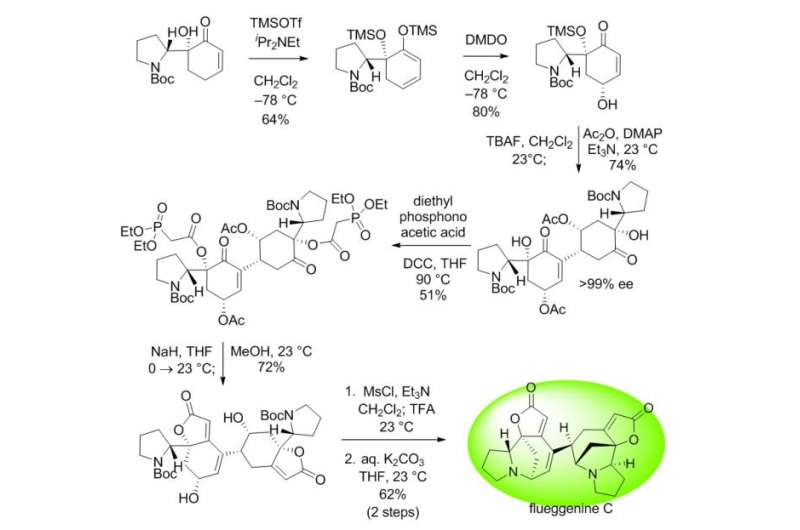
The conventional intermolecular RC reaction was driven non-selectively by a toxic nucleophilic catalyst at a high temperature of over 150°C and a highly concentrated reaction mixture, and thus has never been applied to natural products total synthesis. To overcome this long-standing problem, the research team placed a nucleophilic moiety at the y-position of the enone derivative. As a result, the RC reaction could be induced by the simple addition of a base at ambient temperature and dilute solution, without the need of a nucleophilic catalyst. Using this newly discovered reactivity, the team successfully synthesized the natural product (-)-flueggenine C from commercially available amino acid derivative in 12 steps.
Professor Han said, "Our key finding regarding the remarkably improved reactivity and selectivity of the intermolecular RC reaction will serve as a significant stepping stone in allowing this reaction to be considered a practical and reliable chemical tool with broad applicability in natural products, pharmaceuticals, and materials syntheses."
This research was led by Ph.D. candidate Sangbin Jeon and was published in The Journal of the American Chemical Society (JACS) on May 10.
More information: Sangbin Jeon et al, An Accelerated Intermolecular Rauhut–Currier Reaction Enables the Total Synthesis of (−)-Flueggenine C, Journal of the American Chemical Society (2017). DOI: 10.1021/jacs.7b02751
Journal information: Journal of the American Chemical Society