First luminescent molecular system with a lower critical solution temperature

Depending on their solubility, solids can completely dissolve in liquids to form clear solutions, or form suspensions that still contain undissolved solid. Solutions of polymers often have a lower critical solution temperature; only below this temperature is the polymer completely soluble at all concentrations.
However, it is rare for non-polymeric mixtures to have a lower critical solution temperature because small molecules usually become more soluble as they are heated.
Osaka University researchers have now created a mixture of small organic and inorganic molecules that has a lower critical solution temperature. Their luminescent mixture is easily switched from a solution to a suspension and back again, simply by changing the temperature. The system, which has a different emission color depending on whether it is in the solution or suspension state, will be useful for the development of new thermo-responsive materials that change color when heated. The study was recently published in the journal Advanced Materials.
"This behavior is usually only observed in polymer systems," says Associate Professor Akinori Saeki, corresponding author of the study, "because they undergo structural changes at high temperatures that reduce their solubility. This is the first example of a luminescent molecule/ion-based lower critical solution temperature system."
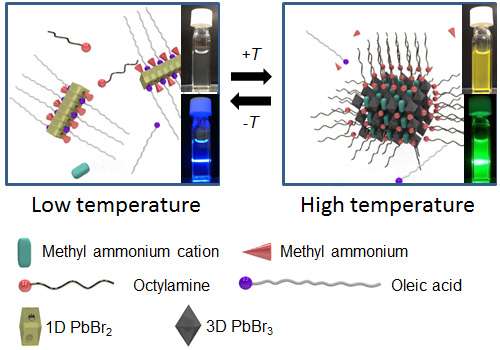
The researchers based their system on methyl ammonium lead bromide nanoparticles, which have been used to develop new-generation LEDs and lasers. Noting that these nanoparticles are reversibly broken apart into their molecular components in the presence of certain amines, the researchers prepared a mixture of the nanoparticles with methylamine and other organic molecules.
At room temperature, the mixture was a clear solution that emitted blue light when it was irradiated under UV light. When the researchers heated this clear solution, however, it became white and cloudy, and then formed a yellow suspension above a critical temperature. The yellow suspension emitted green light when irradiated with UV light.
"Using X-ray diffraction, we found that the clear solution contained soluble 1D wires made up of lead bromide, methylamine and oleic acid," Dr Saeki says. "As the solution was heated, these wires rearranged into a co-crystal containing lead bromide and methylamine, which was insoluble in the solvent."
The intermediate co-crystal was an essential step before formation of the yellow nanoparticles at higher temperatures, and its assembly and fragmentation were mediated by the organic molecules oleic acid and methylamine.
Tuning the system by varying the concentrations of the organic molecules or adjusting the ratio of halide ions (chloride, bromide and iodide) in the nanoparticles, the researchers have developed a series of multicolored systems with the same luminescent behavior, and hope to use them in new-generation photomaterials.
More information: Ryosuke Nishikubo et al. Thermoresponsive Emission Switching via Lower Critical Solution Temperature Behavior of Organic-Inorganic Perovskite Nanoparticles, Advanced Materials (2017). DOI: 10.1002/adma.201700047
Journal information: Advanced Materials
Provided by Osaka University