Closing the gate to mitochondria

Eukaryotic cells contain thousands of proteins, which are distributed to different cellular compartments with specific functions. A German-Swiss team of scientists led by Prof. Dr. Bettina Warscheid from the University of Freiburg and Prof. Dr. André Schneider from the University of Bern has developed the method "ImportOmics". This method enables the scientists to determine the localization of proteins that are imported via specific entry "gates" into distinct membrane-bound compartments, so-called organelles. Knowing the exact localization of individual proteins, the route they take to reach their destination, and the overall composition of cellular compartments is important for understanding fundamental mechanisms of cell biology. This is the prerequisite to understand disease mechanisms that rely on defective cellular functions.
The scientists present their work in the current issue of the journal Nature Communications.
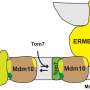
The research team developed the method to define the mitochondrial protein inventory of the single-cell parasite Trypanosoma brucei. The parasite contains a single mitochondrion, which is essential for growth and survival. The mitochondrion is surrounded by two membranes and houses more than thousand proteins. The exact protein composition, however, has not yet been established. The majority of these proteins are synthesized in the cellular fluid, the cytosol, and need to cross the outer membrane of the mitochondrion before they are sorted to their final destination. To this end, the outer membrane is equipped with a central gate, the so-called archaic translocase of the mitochondrial outer membrane (ATOM). The scientists exploited this gate to define the entirety of the mitochondrial proteins imported from the cytosol. They engineered cells to express reduced levels of ATOM40, the pore-forming component of the ATOM complex, thereby blocking the protein import into the mitochondrion.
The research team used quantitative mass spectrometry to compare the levels of proteins in mitochondria with defective and with undisturbed protein import. As a result, the scientists identified 1,120 proteins, including more than 300 proteins that, so far, had not been associated with the mitochondrion of the parasite. In addition, they showed that ImportOmics is applicable to systematically analyze different cellular protein import systems. This is exemplified for the import of proteins into the outer mitochondrial membrane and into the mitochondrial intermembrane space. Furthermore, scientists can use this method to analyze the composition of other organelles of the parasite as well as of other organisms.
More information: Christian D. Peikert et al, Charting organellar importomes by quantitative mass spectrometry, Nature Communications (2017). DOI: 10.1038/NCOMMS15272
Journal information: Nature Communications
Provided by University of Freiburg