Energy-efficient green route to magnesium production
A research group led by Professor Yuji Wada and Adjunct Professor Satoshi Fujii of the Tokyo Institute of Technology has devised a magnesium smelting method that uses nearly 70 percent less energy than conventional methods by using microwaves.
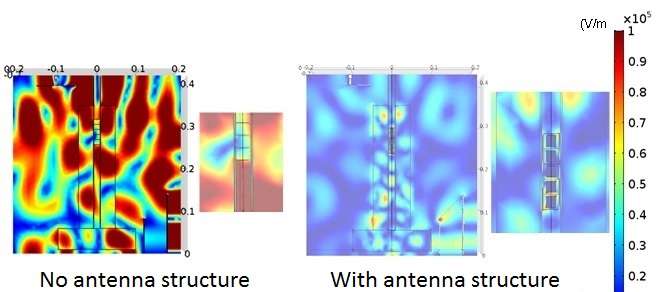
Dolomite ore (MgO, CaO), which is a raw material for magnesium metal, does not absorb microwave energy well and does not generate heat. When electrically conductivity ferrosilicon (FeSi) used as a reducing agent was mixed with the raw dolomite material and made into an antenna structure, it more easily absorbed the microwave energy and reduced in temperature. Internal heating and contact point heating, which are microwave characteristics, were observed, and the average reaction temperature for this smelting was lowered from the conventional 1,200 - 1,400°C to 1,000°C.
This research result was published in the April 12th issue of Scientific Reports.
Currently, the smelting of magnesium metal is mainly performed using the Pidgeon method (a thermal reduction method) in which the material temperature is raised using a large amount of coal as the heat source. About 80 percent of magnesium metal is produced in China. A large amount of coal is consumed for smelting, resulting in the generation of the air pollutant PM 2.5 (fine particulate matter) and the release of carbon dioxide to the atmosphere, which are major problems.
The Pidgeon method is a technique for heating dolomite ore and silicon iron to high temperatures and then cooling the evaporated magnesium to obtain magnesium metal.
Where (s)=Solid and (g)=Gas:
2MgO (s) + 2CaO (s) + (Fe)Si (s) → 2Mg (g) + Ca2SiO4 (s)+ Fe (s)
Dolomite mineral: MgO, CaO; Ferrosilicon: FeSi
Heat source: Coal
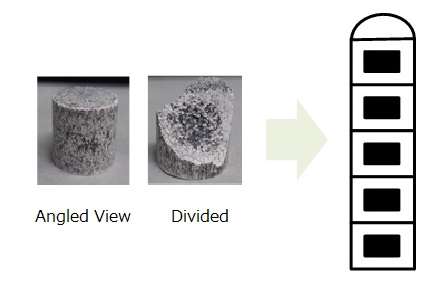
By using ferrosilicon as the reducing agent, devising the shape of the raw material pellet obtained by mixing dolomite and ferrosilicon and forming it as an antenna with a resonance structure of 2.45 GHz (the frequency for microwave ovens), it was possible to confine the microwave energy to the pellet.
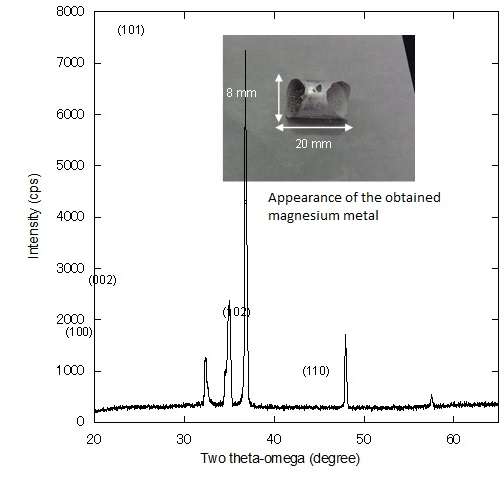
In a small-scale experimental reactor, 1g of magnesium metal was smelted successfully. Also, in order to accurately estimate the energy, a demonstration furnace about five times larger than the experimental furnace was produced and experiments were conducted, resulting in the successful smelting of about seven grams of magnesium metal. This can reduce energy by 68.6 percent compared with the conventional method.
Future Developments
This technique is promising for the high-temperature reduction process of oxides. In the future, through further development of this research, it will be applied to the smelting of other metal materials to save energy with steel, metals, materials and chemistry, and reduce carbon dioxide pollution, which is one of the causes of global warming.
More information: Yuji Wada et al, Smelting Magnesium Metal using a Microwave Pidgeon Method, Scientific Reports (2017). DOI: 10.1038/srep46512
Journal information: Scientific Reports
Provided by Tokyo Institute of Technology