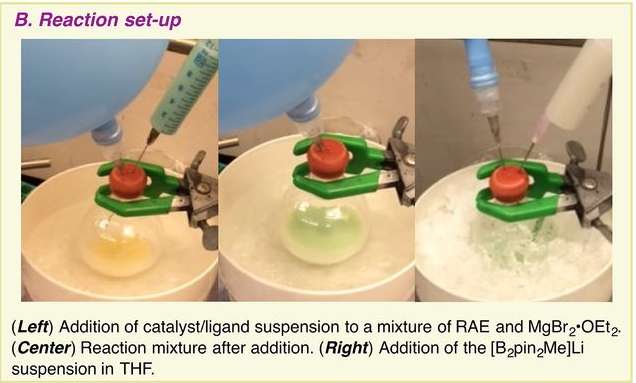
Credit: (c) Science (2017). DOI: 10.1126/science.aam7355
(Phys.org)—A team of researchers from the Scripps Research Institute has developed a simple, practical method for converting carboxylic acids into boronate esters and boronic acids. In their paper published in the journal Science, the team describes the process and why they believe it will make such compounds more accessible.
Carboxylic acids are acids that belong, quite naturally, to the carboxyl group, which consists of double-bonded carbon atoms to a single oxygen atom and singly bonded to a member of a hydroxyl group. They are used in a variety of manufacturing processes, most often as part of a reaction that produces other compounds such as boronate esters and boronic acids (alkyls or aryl substituted boric acids that have a carbon–boron bond). End products include things like soaps, soft drinks, food products and some drugs, such as the cancer therapy Velcade. But as the researchers note, until now, the process has involved converting such acids to more useful compounds in ways they describe as non-trivial. In this new effort, the researchers describe a technique to convert such acids to boronate esters and boronic acids that is practical and relatively simple.
To convert a carboxylic acid to a boron compound, the researchers started by converting it to a redox-active N-hydroxyphthalimide ester. A nickel catalyst was then used to induce decarboxylative borylation in the presence of bis(pinacolato)diboron, a bipyridine ligand to produce a boronate ester, which was then hydrolyzed. They note the technique works equally well on a host of tertiary carboxylic acids and even peptides. They also point out that it can be used in late-stage transformations of complex molecules, making it useful in a particularly broad range of applications.
To prove its value, the team worked with a group at Calibr, and together they used the technique to create analogs of human neutrophil elastase inhibitors, which are used to treat lung diseases. Calibr has been looking for a way overcome potency issues, and report that the new technique resulted in compounds that were much more potent than those made the traditional way.
The new technique is relatively straightforward, which means it will likely be tried and adapted first in laboratories around the world, and then put into production processes relatively quickly, possibly reducing costs.
More information: Chao Li et al. Decarboxylative borylation, Science (2017). DOI: 10.1126/science.aam7355
Abstract
The widespread use of alkyl boronic acids and esters is frequently hampered by the challenges associated with their preparation. Herein we describe a simple and practical method to rapidly access densely functionalized alkyl boronate esters from abundant carboxylic substituents. This broad-scope Ni-catalyzed reaction uses the same activating principle as amide bond formation to replace a carboxylic acid with a boronate ester. Application to peptides allowed expedient preparations of α-amino boronic acids, often with high stereoselectivity, facilitating the synthesis of both FDA approved alkyl boronic acid drugs (Velcade and Ninlaro) as well as a boronic acid version of the iconic antibiotic vancomycin. The reaction also enabled the discovery and extensive biological characterization of potent elastase inhibitors which may have a strategic advantage due to their covalent-reversible binding properties.
Journal information: Science
© 2017 Phys.org