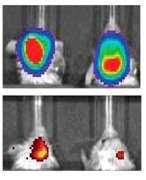
The top row indicates mice with glioblastoma controlled for tumor size. The bottom row shows their expression of MGMT, a protein that makes tumor cells more resistant to chemotherapy. The left is a control, while the right shows significantly decreased levels of MGMT after treatment with the nanoparticles. Credit: Northwestern University
Northwestern Medicine scientists have developed a novel testing platform to assess, in real time, the efficacy of nanomaterials in regulating gene expression. The findings, published in Proceedings of the National Academy of Sciences, could help to facilitate preclinical investigations and optimize nanotherapeutics for cancers before they reach clinical trials.
Timothy Sita, a seventh-year MD/PhD student in the Medical Scientist Training Program, was the first author of the study, which looked at the platform in animal models.
"This is an important step forward for the field," said principal investigator Alexander Stegh, PhD, assistant professor of Neurology and of Medicine. "The very thorough optimization that we see in conventional drug development had been missing in the nanotech space, and we felt very strongly about changing this. The system that we developed here really allows us to support those efforts, and evaluate our nanoparticles in the most relevant models, in an in vivo setting."
Chad Mirkin, PhD, the George B. Rathmann Professor of Chemistry in the Weinberg College of Arts and Sciences and a professor of Medicine in the Division of Hematology/Oncology, was also a corresponding author of the paper.
The scientists demonstrated the concept while using nanostructures called spherical nucleic acids (SNAs) to target a resistance factor gene in glioblastoma, an aggressive, incurable type of brain tumor.
SNAs, first developed by Mirkin at Northwestern in 1996, consist of dense strands of RNA packed around a nanoparticle core. Because of their unique properties, SNAs are capable of both crossing the blood-brain barrier and entering into tumor cells, where they can directly target gene activity that encourages cancer growth.
While these conjugates are a promising tool in the era of precision medicine, scientists previously lacked a quantitative method to assess how SNAs regulated gene activity in living organisms, which would provide new insights into how to optimize the therapies.
"We've seen that these particles can basically target any cancer gene, but we didn't know when they worked best or what dosing regimens to use," Sita said. "As such, preclinical trials weren't as successful as they could have been."
In the current study, the scientists showed that by using a type of non-invasive imaging on the mice, they could gauge in real time how the nanoparticles affected levels of an intratumoral target protein.
"Now we can tweak these particles—play with the shape of the nanoparticle, or how much RNA we load onto the particle, for example—and then assess very quickly whether those changes are more effective or not," Sita explained. "It's a platform to help optimize the drugs in mice before they go to human trials, and make something that will translate better to the clinic."
While the method could be generalizable to investigating nanotherapeutics for many types of cancers, the study also has clinical implications unique to glioblastoma.
The scientists developed nanoparticles to knock down O6-methylguanine-DNA methyltransferase (MGMT)—a protein which reduces the impact of chemotherapy—in mice with glioblastoma. Through the imaging platform, they discovered that mice had the lowest levels of the protein between 24 to 48 hours after receiving the nanoparticles, suggesting the optimal time to administer chemotherapy.
"We showed a very significant reduction in tumor volume when we combined these particles with the chemotherapy," Sita said. "By silencing this gene that's causing resistance to the chemotherapy, we can have a much more profound response. That's the key clinical angle."
More information: Timothy L. Sita et al. Dual bioluminescence and near-infrared fluorescence monitoring to evaluate spherical nucleic acid nanoconjugate activity in vivo, Proceedings of the National Academy of Sciences (2017). DOI: 10.1073/pnas.1702736114
Journal information: Proceedings of the National Academy of Sciences
Provided by Northwestern University