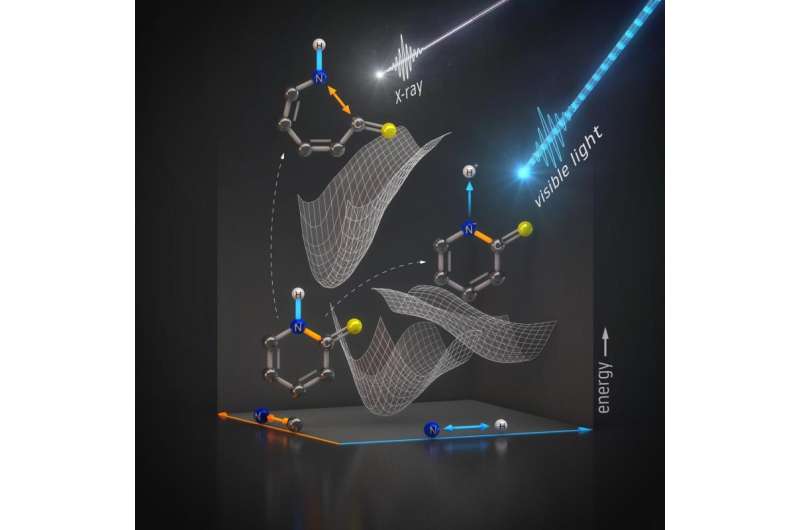
An illustration shows how energy from light changes a 2-thiopyridone molecule. Optical light strikes the molecule, and it loses a proton bound to hydrogen. An X-ray probe allows scientists to follow selective bond breaking between nitrogen and its carbon and hydrogen neighbors. Credit: Helmholtz-Zentrum Berlin
When the molecules that carry the genetic code in our cells are exposed to harm, they have defenses against potential breakage and mutations.
For instance, when DNA is hit with ultraviolet light, it can lose excess energy from radiation by ejecting the core of a hydrogen atom—a single proton—to keep other chemical bonds in the system from breaking.
To gain insight into this process, researchers used X-ray laser pulses from the Linac Coherent Light Source (LCLS) at the Department of Energy's SLAC National Accelerator Laboratory to investigate how energy from light transforms a relatively simple molecule, 2-thiopyridone. This molecule undergoes a chemical transformation that also occurs in the building blocks of DNA. The scientists looked at this process by probing the nitrogen atom in the molecule with X-ray pulses that lasted just femtoseconds, or quadrillionths of a second.
The results, published in Angewandte Chemie, are a step toward better understanding what's called "excited state proton transfers" in DNA and other molecules.
"Right now, we want to keep it simple," says lead author Sebastian Eckert, a doctoral student at the University of Potsdam and Helmholtz-Zentrum Berlin. "It's easier to look at the effects of photoexcitation in 2-thiopyridone because this molecule is small enough to understand and has only one nitrogen atom. We are among the first at LCLS to look at nitrogen at this energy, so it's somewhat of a pilot experiment."
This is also the first time the method, known as resonant inelastic X-ray scattering or RIXS, has been used to look at molecular changes involving nitrogen that happen in femtoseconds. This short timescale is important because that's how fast protons are kicked away from molecules exposed to light, and it requires brilliant X-rays to see these ultrafast changes.
"LCLS is the only X-ray light source that can provide enough photons – particles of light," says co-author Munira Khalil, a professor at the University of Washington. "Our detection mechanism is 'photon-hungry' and requires intense pulses of light to capture the effect we want to see."
In the study, the researchers used an optical laser to initiate changes in the molecule, followed by an LCLS X-ray probe that allowed them to see movements in the bonds.
"We look for a resonance effect – a signature that lets us know we've tuned the X-rays to an energy that ensures we're only examining changes related to or near the nitrogen atom," says Mike Minitti, staff scientist at LCLS and co-author of the paper.
These "on-resonance" studies amplify the signal in a way that scientists can clearly interpret how X-rays interact with the sample.
The research team looked primarily at the bonds between atoms neighboring nitrogen, and confirmed that optical light breaks nitrogen-hydrogen bonds.
"We were also able to confirm that the X-rays used to probe the sample don't break the nitrogen-hydrogen bond, so the probe itself does not create an artificial effect. The X-ray energy is instead transferred to a bond between nitrogen and carbon atoms, rupturing it," says Jesper Norell, a doctoral student at Stockholm University and co-author of the paper.
Next, the collaboration will use the same approach to study more complex molecules and gain insight into the wide class of photochemical reactions.
More information: Sebastian Eckert et al. Ultrafast Independent N−H and N−C Bond Deformation Investigated with Resonant Inelastic X-Ray Scattering, Angewandte Chemie International Edition (2017). DOI: 10.1002/anie.201700239
Journal information: Angewandte Chemie International Edition , Angewandte Chemie
Provided by SLAC National Accelerator Laboratory