This is a better way to make diaryl ether. Credit: Waseda University
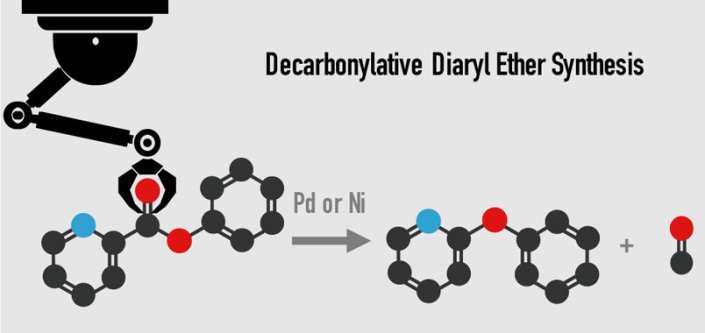
A group of Waseda University researchers has developed a new process using palladium or nickel as a catalyst for removing carbon monoxide from esters to produce ethers. This innovation provides new opportunities for development of drugs to fight cancer, malaria and more.
The conventional method for producing diaryl ether uses an intermolecular cross-coupling reaction of aryl halides and phenols with a copper or palladium catalyst, but high cost and concerns about disposal of potentially hazardous halogenated waste have driven demand for a better method.
In this research, a nickel or palladium catalyst with an enabling diphosphine ligand successfully removed carbon monoxide from aromatic esters to synthesize diaryl ether. Using this innovative process, diaryl ethers can be produced from over 30 different kinds of aromatic esters, allowing a choice of more inexpensive and easily obtainable materials. The present reaction can also be conducted on a gram scale with excellent yield, all of which is expected to make a significant impact on development of new pharmaceuticals.
More information: Ryosuke Takise et al, Decarbonylative Diaryl Ether Synthesis by Pd and Ni Catalysis, Journal of the American Chemical Society (2017). DOI: 10.1021/jacs.7b00049
Journal information: Journal of the American Chemical Society
Provided by Waseda University