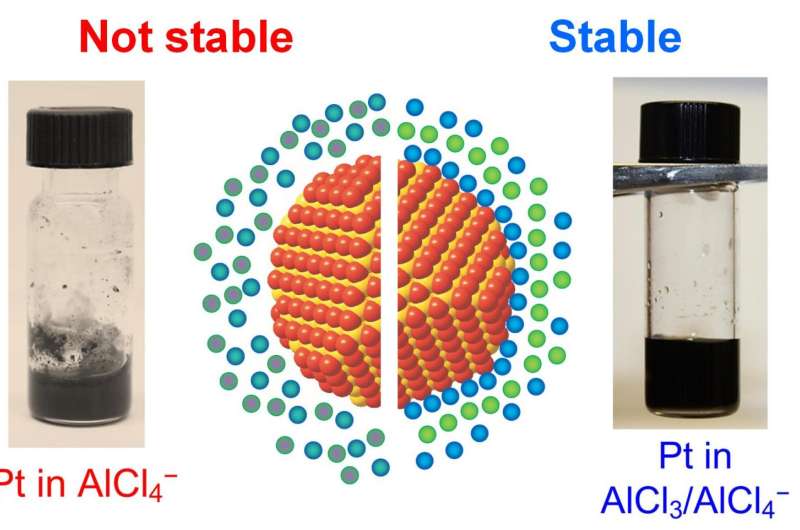
Chemical bonds between the nanoparticle surface (represented by orange spheres) and species in molten salt (represented by blue and green spheres) are necessary for colloidal stability. Corresponding examples of stable and unstable colloidal dispersions of platinum nanocrystals are shown in the photographs. Sstable solution: Pt nanocrystals in AlCl3/NaCl/KCl molten salt containing excess of AlCl3 that bind to nanocrystal surface. Unstable solution: same nanocrystals in AlCl3/NaCl/KCl molten salt where the composition was adjusted to contain only chemically inert Na+, K+, and AlCl4- ions. Credit: Dmitri Talapin
(Phys.org)—Colloidal systems are important in nanoscience and materials. A colloidal system involves the dispersion of particles within a solvent. A stable colloid has evenly dispersed solute particles while unstable colloids form aggregates of solute particles. To prevent aggregation, researchers can functionalize the surface of the solute particles so that the surface matches the polarity of the solvent but repels neighboring particles.
Researchers from the University of Chicago, the University of Massachusetts, and Argonne National Laboratory have developed stable colloids made of nanocrystals solute particles in a solvent of molten inorganic salts. The solutes include metals, semiconductors, and magnetic materials. Their surface was modified to match the properties of the inorganic salts. Furthermore, screening studies and computational analyses showed that colloidal stability was based on the strength of the chemical bond at the solute-solvent interface. Their work appears in Nature.
"Molten salts are unusual solvents made up exclusively of small inorganic ions," principle investigator Dmitri Talapin told Phys.org. "They differ considerably from traditional solvents used to disperse colloids such as water or organic solvents."
Hao Zhang, Kinjal Dasbiswas, Nicholas B. Ludwig, Gang Han, Byeongdu Lee, Suri Vaikuntanathan, and Dmitri V. Talapin developed several nanocrystal solute particles that are comprised of semiconductors, rare-earth compounds, and magnetic materials that were originally functionalized with hydrophobic organic molecules (e.g., oleate). After surface modifications, these nanoparticles were dispersed in a molten solution of various inorganic salts, all of which had melting points below 350oC. Many of the inorganic salts tested were combinations of metal halides or isocyanates or aluminum bromide.
Platinum nanocrystals form a stable colloid in the eutectic mixture of molten AlCl3/NaCl/KCl. Credit: Dmitri Talapin
Structural studies using small-angle x-ray scattering, x-ray diffraction, transmission electron microscopy, IR spectroscopy, and UV-visible spectroscopy showed that nanocrystals dispersed in the molten inorganic salts without sedimentation or aggregation. The reason behind the formation of stable colloids, however, needed to be investigated as typical repulsive interactions such as Coulombic forces or sterics were not in play here.
Experimental studies showed that stable colloids tended to form when there was a chemical affinity between the nanocrystal surface and the solvent ions. For example, platinum nanocrystals dispersed in salts containing excess AlCl3, or displaying Lewis acidity, to form a stable colloid. However, in a solvent that does not display Lewis acidity or basicity, the platinum nanocrystals formed aggregates.
Computational studies using CdSe nanocrystals in molten KCl together with a theoretical analysis of the Coulombic and steric interactions between solvent ions showed that ionic solvent structuring occurs at the solute-molten salt interface. This ion layering in the solvent served to prevents aggregates from forming.
According to Dr. Talapin, their work "demonstrates that colloidal nanoparticles can be stabilized in this unusual solvent and that the strong interactions between ions provide a novel mechanism for colloidal stabilization."
This research demonstrates a new type of colloidal system that has possible utility in the area of nanoscience and materials. Additional studies in this area could include looking at more exotic solvents beyond molten inorganic salts. Also the size and shape of the nanoparticles can be tailored for heat transfer and thermal storage. The high temperature stability of inorganic salts can be utilized for colloidal synthesis of novel nanoparticles.
More information: Hao Zhang et al. Stable colloids in molten inorganic salts, Nature (2017). DOI: 10.1038/nature21041
Abstract
A colloidal solution is a homogeneous dispersion of particles or droplets of one phase (solute) in a second, typically liquid, phase (solvent). Colloids are ubiquitous in biological, chemical and technological processes1, 2, homogenizing highly dissimilar constituents. To stabilize a colloidal system against coalescence and aggregation, the surface of each solute particle is engineered to impose repulsive forces strong enough to overpower van der Waals attraction and keep the particles separated from each other2. Electrostatic stabilization3, 4 of charged solutes works well in solvents with high dielectric constants, such as water (dielectric constant of 80). In contrast, colloidal stabilization in solvents with low polarity, such as hexane (dielectric constant of about 2), can be achieved by decorating the surface of each particle of the solute with molecules (surfactants) containing flexible, brush-like chains2, 5. Here we report a class of colloidal systems in which solute particles (including metals, semiconductors and magnetic materials) form stable colloids in various molten inorganic salts. The stability of such colloids cannot be explained by traditional electrostatic and steric mechanisms. Screening of many solute–solvent combinations shows that colloidal stability can be traced to the strength of chemical bonding at the solute–solvent interface. Theoretical analysis and molecular dynamics modelling suggest that a layer of surface-bound solvent ions produces long-ranged charge-density oscillations in the molten salt around solute particles, preventing their aggregation. Colloids composed of inorganic particles in inorganic melts offer opportunities for introducing colloidal techniques to solid-state science and engineering applications.
Journal information: Nature
© 2017 Phys.org