Heavy metal binding domain in a cysteine-rich protein may be sea snail adaptation to metal stress
A special type of small sulfur-rich protein, metallothioneins, have an extraordinary capability for binding heavy metals. An international team of scientists has now discovered that the marine common periwinkle, which is widely considered a delicacy, contains the largest version of the protein found yet, with one additional cadmium-binding domain and a one-third higher detoxification capacity. As they report in the journal Angewandte Chemie, this feature may help the snail survive in heavy-metal-polluted environments.
Snails and slugs are known for their intriguing ability to accumulate and detoxify heavy metals. They are even capable of discriminating between cadmium and copper, as the latter element is an indispensable element in their metabolism, while cadmium is toxic. They detoxify cadmium by binding it to metallothioneins, a class of small proteins rich in the sulfur-containing cysteine amino acid. Oliver Zerbe at the University of Zurich, Switzerland, and Reinhard Dallinger at the University of Innsbruck, Austria, and their colleagues in Barcelona, Spain, investigate the evolution of these proteins as a strategy to adapt the gastropodes to their new habitats—land snails have developed from marine species, and had to find novel strategies to cope with the higher loadings of heavy metals in the soil. Still harsher environments are found at the sea shores with their fluctuating water supply. As the scientists discovered, the marine gastropod Littorina littorea (common periwinkle), which has very successfully colonized the Northern Atlantic shores, has found a peculiar strategy for even more efficient detoxification.
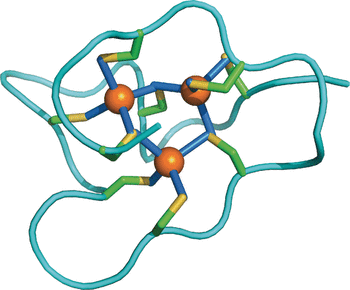
Studying the molecular differences between the proteins among various species, the scientists solved the solution structure of the periwinkle metallothionein by using nuclear magnetic resonance techniques and compared it with other known structures and sequences. Surprisingly, the periwinkle's protein comprises three independent domains, while other known metallothioneins have only one or two. Each of the three domains contains nine cystein residues binding a cluster of three cadmium ions, thus the total 27 cystein residues can incorporate nine cadmium ions. This sheds light on the adaptation strategy: "Increasing the number of domains simply increases the metal-binding capacity of the protein and thereby potentiates its metal-detoxification capacity," the authors wrote.
Regarding the structural features, the complex formed with cadmium is very similar to that of the Roman snail form, which can efficiently discriminate between the copper ions vital to snails and toxic cadmium. And apart from simply coping with cadmium-rich environments and selecting copper from other heavy metals, the cysteine-rich metallothioneins are considered as important oxidative-stress-response proteins. Stress is one of the major encounters of the common periwinkle, which can survive rough seas and drought at the same time.
More information: Christian Baumann et al. Structural Adaptation of a Protein to Increased Metal Stress: NMR Structure of a Marine Snail Metallothionein with an Additional Domain, Angewandte Chemie International Edition (2017). DOI: 10.1002/anie.201611873
Journal information: Angewandte Chemie International Edition , Angewandte Chemie
Provided by Wiley