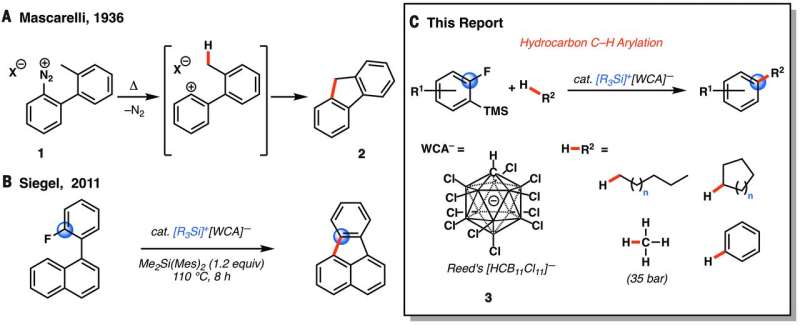
Reactions involving putative phenyl cations. (A) Mascarelli’s reaction (12). (B) Siegel’s intramolecular Friedel-Crafts reaction of aryl fluorides (15). (C) Our dual C–F/C–H functionalization strategy. R = ethyl or triisopropyl; R1 = aryl, alkyl, halide, or silyl ether; WCA, weakly coordinating anion; TMS, trimethylsilyl; Mes, mesityl; Me, methyl; cat., catalytic. Credit: (c) Science (2017). DOI: 10.1126/science.aam7975
(Phys.org)—A team of chemists at the University of California has developed a cheaper way to functionalize unactivated alkanes (hydrocarbons such as ethane, methane and propane) by using much more abundant catalysts. In their paper published in the journal Science, the team describes the reaction design they created that overcomes prior challenges related to high-energy reactivity profiles.
Currently, unactivated alkanes are considered difficult to functionalize—most use an approach that involves opening hydrocarbon C-H bonds using a process involving precious and expensive metals. In this new effort, the team has found a way to use silicon and boron as catalysts instead. This is important because of the industry need to convert alkanes such as natural gas and petroleum to more valuable products.
Alkanes do react relatively easily at high temperatures, as is seen in internal combustions engines, but such reactions are considered to be extremely difficult to control. Functionalizing alkanes allows for gaining more from them than just their energy potential, it allows for extracting components that can be used as precursors for the creation of rare chemicals which, in some cases, are considerably more valuable. Because they are relatively inert, and the bonds between the carbon and hydrogen are strong, they are difficult to functionalize. To break those strong bonds, the team made even stronger ones.
The group started with benzene rings, preparing them with silicon and fluorine substitutes. They next primed a cycle using additional activated silicon, which they paired with carborane—this forced the fluorine to create an aryl intermediate which was able to break the C-H alkane bonds (which notably included methane). The resultant ring then released silicon, which kept the reaction proceeding. The team notes that the reaction can be conducted under temperature ranges from 30° to 100°C. It is not yet clear whether the process can be scaled to be cost effective, but if so, it could lead to a drop in prices for some products, particularly those based on increasingly abundant natural gas.
The group is scheduled to give a presentation regarding their work at the next ACS meeting.
More information: Brian Shao et al. Arylation of hydrocarbons enabled by organosilicon reagents and weakly coordinating anions, Science (2017). DOI: 10.1126/science.aam7975
Abstract
Over the past 80 years, phenyl cation intermediates have been implicated in a variety of C–H arylation reactions. Although these examples have inspired several theoretical and mechanistic studies, aryl cation equivalents have received limited attention in organic methodology. Their high-energy, promiscuous reactivity profiles have hampered applications in selective intermolecular processes. We report a reaction design that overcomes these challenges. Specifically, we found that β-silicon–stabilized aryl cation equivalents, generated via silylium-mediated fluoride activation, undergo insertion into sp3 and sp2 C–H bonds. This reaction manifold provides a framework for the catalytic arylation of hydrocarbons, including simple alkanes such as methane. This process uses low loadings of Earth-abundant initiators (1 to 5 mole percent) and occurs under mild conditions (30° to 100°C).
Journal information: Science
© 2017 Phys.org