Technique to suppress dendrite growth in lithium metal batteries
A team of researchers at Tsinghua University (Beijing, China) has reported an implantable solid electrolyte interphase (SEI) in a lithium metal anode. This stable SEI film can suppress lithium dendrite growth to realize safe lithium metal batteries (LMB). Their results appear in the journal Chem, published on February 9, 2017.
"Lithium metal has been regarded as a 'holy grail' electrode owing to its lowest electrode potential (-3.040 V vs standard hydrogen electrode) and high capacity (3860 mAh g-1). It is thus under extensive investigation. Consequently, LMBs are considered as promising next-generation energy storage systems," said Qiang, the corresponding author. "The uncontrolled growth of Li dendrites, however, leads to the dilemma of fluctuating Coulombic efficiency and formidable safety issues, unfortunately preventing the commercialization of LMBs."
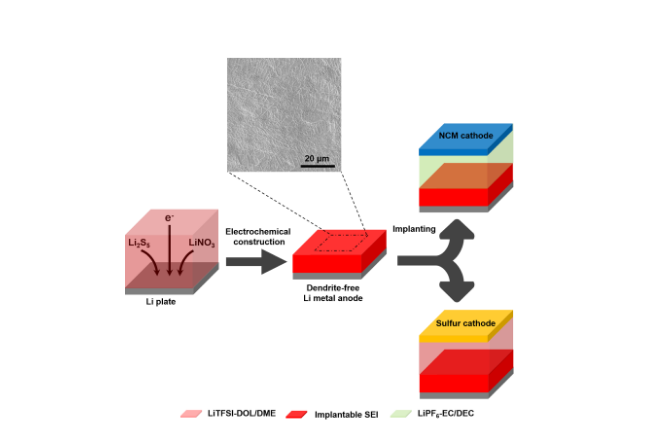
Lithium dendrites are caused by unstable electrode/electrolyte interfaces and the induced inhomogeneous distribution of electrons and Li ions on the anode surface. "The dual-salt additive including the Li polysulfide (LiPS) and LiNO3 were discovered to be effective for constructing a dense and stable SEI to suppress lithium dendrite growth in the ether electrolyte (the commonly used sulfur cathode electrolyte). However, this additive fails with the lithium-rich cathode, because it can only work well in the ester electrolyte, which can react with LiPS heavily. To obtain a universal strategy to protect the lithium metal anode, we achieved an implantable SEI film on the lithium metal using electroplating methods in the LiPS/LiNO3 electrolyte," said Xin-Bing Cheng, the first author of this work, a graduate student at Qiang's group in Tsinghua University.
The resulting implantable SEI film works well with the sulfur cathode in the ether electrolyte, and also the lithium-rich LiNi0.5Co0.2Mn0.3O2 (NCM) cathode. Lithium metal with the implantable SEI can effectively suppress lithium dendrite growth and improve the lifespan of LMB. Chong Yan, co-first-author, said, "The implantable SEI can increase short circuit time by 83 percent (from 3 to 5.5 hr) to suppress Li dendrite growth. Li-S pouch cells with the advanced lithium metal anode exhibited improved discharge capacity from 156 to 917 mAh g-1 and enhanced Coulombic efficiency from 12 to 85 percent at 0.1 C by remarkably protecting the lithium metal and inhibiting shuttle of lithium polysulfide intermediates. The electroplated implantable SEI can be extended to match NCM cathode in the carbonate ester-based electrolyte. At a low rate of 18.2 mA g-1, COM cell indicates a severe decay of Coulombic efficiency from 97 to 82 percent, while the advanced cell can maintain 99 percent stably. The superb performance without any activation strongly confirms the highly conductive nature of electroplated SEI."
"The electroplated implantable SEI here not only provides a creative method to construct a stable protection for the lithium metal anode, but also makes it possible to obtain a lithium metal anode by compatibly coupling the superiority of SEI formed in different electrolytes. The highly conductive and stable implantable SEI on the lithium metal anode opens a novel branch of interfacial chemistry and a material platform for advanced energy storage systems using metal electrodes," Qiang said.
More information: Xin-Bing Cheng et al. Implantable Solid Electrolyte Interphase in Lithium-Metal Batteries, Chem (2017). DOI: 10.1016/j.chempr.2017.01.003
Provided by Tsinghua University