Credit: American Chemical Society
Wearable electronics are here—the most prominent versions are sold in the form of watches or sports bands. But soon, more comfortable products could become available in softer materials made in part with an unexpected ingredient: green tea. Researchers report in ACS' The Journal of Physical Chemistry C a new flexible and compact rechargeable energy storage device for wearable electronics that is infused with green tea polyphenols.
Powering soft wearable electronics with a long-lasting source of energy remains a big challenge. Supercapacitors could potentially fill this role—they meet the power requirements, and can rapidly charge and discharge many times. But most supercapacitors are rigid, and the compressible supercapacitors developed so far have run into roadblocks. They have been made with carbon-coated polymer sponges, but the coating material tends to bunch up and compromise performance. Guruswamy Kumaraswamy, Kothandam Krishnamoorthy and colleagues wanted to take a different approach.
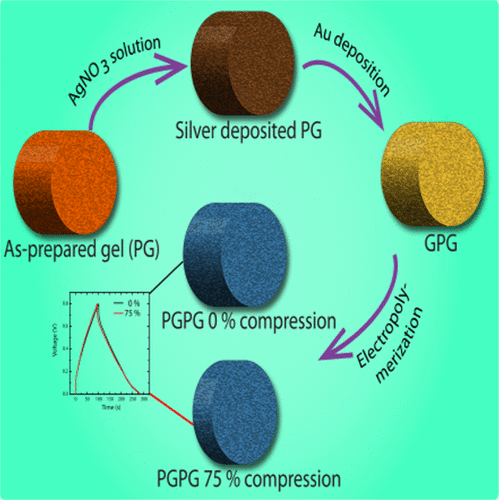
The researchers prepared polymer gels in green tea extract, which infuses the gel with polyphenols. The polyphenols converted a silver nitrate solution into a uniform coating of silver nanoparticles. Thin layers of conducting gold and poly(3,4-ethylenedioxythiophene) were then applied. And the resulting supercapacitor demonstrated power and energy densities of 2,715 watts per kilogram and 22 watt-hours per kilogram—enough to operate a heart rate monitor, LEDs or a Bluetooth module. The researchers tested the device's durability and found that it performed well even after being compressed more than 100 times.
More information: Chayanika Das et al. Elastic Compressible Energy Storage Devices from Ice Templated Polymer Gels treated with Polyphenols, The Journal of Physical Chemistry C (2017). DOI: 10.1021/acs.jpcc.6b12822
Abstract
Design and fabrication of rechargeable energy storage devices that are robust to mechanical deformation is essential for wearable electronics. We report the preparation of compressible supercapacitors that retain their specific capacitance after large compression and that recover elastically after at least a hundred compression–expansion cycles. Compressible supercapacitors are prepared using a facile, scalable method that readily yields centimeter-scale macroporous objects. We ice template a solution of polyethylenimine in green tea extract to prepare a macroporous cross-linked polymer gel (PG) whose walls are impregnated with green tea derived polyphenols. As the PG is insulating, we impart conductivity by deposition of gold on it. Gold deposition is done in two steps: first, silver nanoparticles are formed on the PG walls by in situ reduction by polyphenols and then gold films are deposited on these walls. Gold coated PGs (GPGs) were used as electrodes to deposit poly(3,4-ethylenedioxythiophene) as a pseudocapacitive material. The specific capacitance of PEDOT coated GPGs (PGPG) was found to be 253 F/g at 1 A/g. PGPG could be compressed and expanded over a hundred cycles without any suffering mechanical failure or loss of capacitative performance. The capacitance was found to be 243 F/g upon compressing the device to 25% of its original size (viz. compressive strain = 75%). Thus, even large compression does not affect the device performance. This device shows power and energy densities of 2715 W/kg and 22 Wh/kg, respectively, in the uncompressed state. The macroporous nature of PGPG makes it possible to fill the PGPG pores with gel electrolyte. We report that the gel electrolyte filled supercapacitor exhibited a specific capacitance of 200 F/g, which increased by 4% upon 75% compression.
Journal information: Journal of Physical Chemistry C
Provided by American Chemical Society