February 10, 2017 report
Liposomes modified with temperature-responsive polymers are tuned for cellular uptake
(Phys.org)—Drug delivery is tricky because the therapeutic compound needs to be non-toxic and deliver the correct dosage at the correct time. Some therapeutics are chemically unstable and others do not have the correct solubility profile for cellular uptake. One way that researchers have overcome some of these drawbacks is using stimuli-responsive polymers.
In a research paper in ACS Omega, Jian Wang, Eri Ayano, Yoshie Maitani, and Hideko Kanazawa of Keio University in Japan report the synthesis of the temperature-responsive polymer poly(N-isopropylacrylamide)-co-N,N'-dimethylaminopropylacrylamide (P(NIPAAm-co-DMAPAAm)) and analyzed liposomes modified with this polymer. They found that their polymer undergoes dehydration at around 40oC and that temperature-responsive polymer-modified liposomes had faster cellular uptake and release compared to nonmodified liposomes.
Researchers have been interested in finding ways to modify liposomes, hollow spheres comprised of phospholipid bilayers, so that they can be a more effective drug delivery system. One way is to modify the surface of liposomes with polymers that respond to certain environmental stimuli, such as temperature.
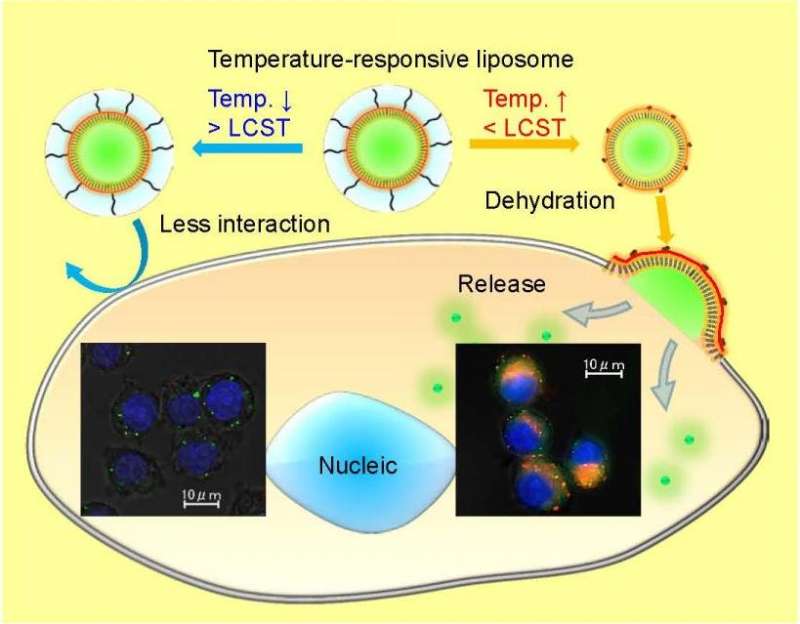
A key factor in liposomes modified with temperature-dependent polymers is the temperature at which its solubility profile changes, known as the lower critical solution temperature (LCST). Below this temperature, the polymers are soluble in an aqueous solution, while above this temperature they become hydrophobic. This causes the polymer to become dehydrated and to aggregate. This behavior guides the release of a drug within a polymer-modified liposome.
In this study Wang et al. synthesized the temperature-responsive polymer poly(N-isopropylacrylamide)-co-N,N-dimethylaminopropylacrylamide (P(NIPAAm-co-DMAPAAm)). NIPAAm is temperature responsive and DMAPAAm is hydrophilic. They then evaluated the LCST of this polymer by looking at the transition of PNIPAAm from a coil configuration to a globular shape after it is dehydrated using differential scanning calorimetry and transmittance curves. They found that the copolymer's LCST was about 40oC.
They then tested this polymer as a modification to the surface of a liposome, DOTAP/DOPE, and compared this to PEGylated liposomes. After optimizing for stability, they studied colloidal stability by looking at particle size in a suspension. They found that as temperature increased, the particle size remained constant until about 39oC. Above 40oC, the particle size increased started forming aggregates.
Studies with carboxyfluorescein (CF) encased in the liposome showed that CF was released once aggregates started to form. Specifically, above 37oC 15% of CF was released in thirty minutes. Then, near the LCST, more CF was released and at 42oC 80% of CF had been released.
Wang et al. then studied changes in the fixed aqueous layer thickness (FALT) on the surface of the polymer-modified liposome and compared it to PEGylated liposomes. These results showed that as the temperature increased, the aqueous layer decreased in size only in the temperature-responsive polymer-modified liposomes, but not in the PEGylated liposomes. Importantly, the aqueous layer's thickness decreased at the LCST.
The next step was to see if the temperature-responsive polymer-modified liposomes displayed good cellular uptake. Wang et al. noted that cellular uptake of the polymer-modified liposomes was much more temperature dependent than the PEGylated liposome controls. Fluorescence microscopy showed that CF-entrapped liposomes (rhodamine labeled) in RAW264.7 and HeLa cells released CF in the cells, while cells treated with free CF and liposomes without CF did exhibit the same level of fluorescence within the temperature range. At 40oC, CF fluorescence appeared throughout the cytosol verifying that the polymer-modified liposomes are temperature dependent in terms of cellular uptake and delivery.
The actual mechanism of cellular uptake was analyzed using HeLa cells. The cells were incubated for one hour at 37oC with or without several types of endocytic inhibitors. The cells were then treated with temperature-responsive polymer modified liposomes at either 4oC or 40oC for 30 minutes. Cellular uptake did not occur at 4oC indicating that uptake is an energy-dependent process. Additionally, based on the results from the inhibitors, cellular uptake occurred via microtubule-dependent transport and clathrin-mediated endocytosis, although additional studies will need to be done to gain further insight into the uptake mechanism.
This research makes headway in the area of drug delivery via liposomes modified with stimuli-responsive polymers. The polymers reported here respond to temperatures that are higher than body temperature but still within physiological range. They likely dehydrate at LCST, leading to good cellular uptake and controlled drug release.
More information: Jian Wang et al. Tunable Surface Properties of Temperature-Responsive Polymer-Modified Liposomes Induce Faster Cellular Uptake, ACS Omega (2017). DOI: 10.1021/acsomega.6b00342
Abstract
Drug delivery by nanoparticle carriers has been limited by inefficient intracellular drug delivery. Temperature-responsive poly(N-isopropylacrylamide) (PNIPAAm)-modified liposomes can release their content following heating. In this study, we synthesized the temperature-responsive polymer poly(N-isopropylacrylamide)-co-N,N′-dimethylaminopropylacrylamide (P(NIPAAm-co-DMAPAAm)) and investigated the properties of liposomes modified with P(NIPAAm-co-DMAPAAm) for intracellular drug carriers. The copolymer displayed a thermosensitive transition at a lower critical solution temperature (LCST) that is higher than body temperature. Above the LCST, the temperature-responsive liposomes started to aggregate and release. The liposomes showed a fixed aqueous layer thickness (FALT) at the surface below the LCST, and the FALT decreased with increasing temperature. Above 37 °C, cytosolic release from the temperature-responsive liposomes was higher than that from the PEGylated liposomes, indicating intracellular uptake. Here, we showed that the tunable surface properties of the temperature-responsive polymer-modified liposomes possibly enabled their dehydration by heating, which likely induced a faster cellular uptake and release. Therefore, the liposomes could be highly applicable for improving intracellular drug-delivery carriers.
© 2017 Phys.org