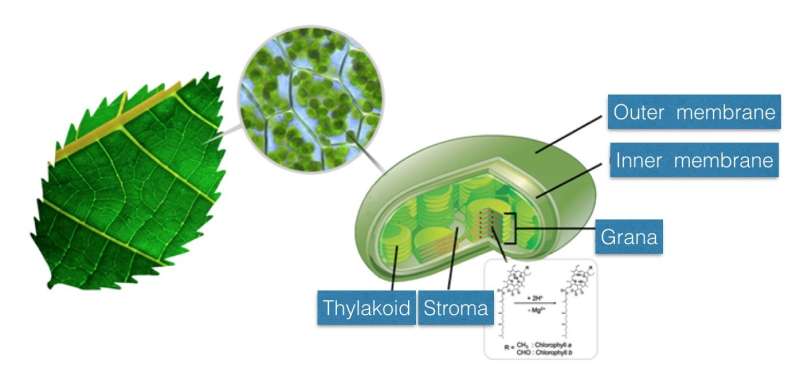
When the chlorophyll encounters an acid, a demetallation reaction occurs in which the magnesium metal ions of the chlorophyll are converted into hydrogen ions. This reaction occurs in many different types of chlorophyll, and the optical, electrical and chemical properties of the chlorophyll are changed by this reaction. Credit: DGIST
DGIST research fellow Hong-Gil Nam and the research team of Professor Richard N. Zere of Stanford University have reported that they have accelerated chlorophyll demetallation by 1000 times over current techniques using microdroplets.
Chlorophyll is a green pigment molecule found in photosynthetic organisms, which plays a key role in the first step of photosynthesis, absorbing light and converting it into chemical energy. In recent years, several research groups have reported accelerated chlorophyll reactions in microdroplets. Evaporation of the solvent or low voltage were cited as the main causes of the acceleration. However, this study conducted experiments challenging existing hypotheses and found that the limitation effect of the physical space of micro-sized droplets is the cause of the accelerated reaction.
The team paid attention to the chemical reaction of chlorophyll in order to reveal the control secret of the absorption and transfer of solar energy. In the acidic condition, the chlorophyll demetallation reaction occurs when the magnesium ions at the center of the chlorophyll are replaced with hydrogen ions. The importance of this reaction in photosynthesis has been overlooked as the reaction rate of the demetallation in bulk solution was very slow compared to the absorption and transfer rate of solar energy in many experiments.
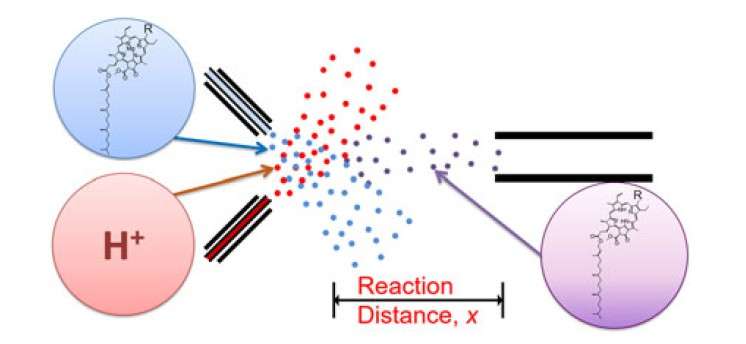
Experimental setup for chlorophyll demetallation kinetics using microdroplet fusion mass spectrometry. Credit: DGIST
In living organisms, a variety of biochemical reactions take place in physically confined spaces. Photosynthesis also occurs in the organelles, the chloroplasts of plants, and the smaller structures called 'grana' in chloroplasts absorb light. The team observed the reaction kinetics of biochemical reactions by creating microdroplets to look at the reaction of chlorophyll in an environment similar to that inside of a plant.
The research team collided water droplets containing chlorophyll with water droplets containing hydrochloric acid at high speed to make micro-sized fused droplets. Then they recorded kinetics of acid-induced chlorophyll demetallation by controlling the traveling distance of the fused microdroplets.
As a result, the researchers found that chlorophyll demetallation occurs within several tens of microseconds, which is about 1000 times faster than the process in bulk solution. This result is presumed to be due to the limitation of the physical space of microdroplets as well as the surface effect of droplets themselves.
Hong-Gil Nam said, "When chlorophyll is oxidized, it loses its photosynthesis function. However, the demetallation reaction can protect the chlorophyll as it prevents the oxidation of chlorophyll."
He added, "This study suggests that the demetallation reaction of chlorophyll can be a new mechanism to protect photosynthetic organisms or control photosynthetic efficiency, and that the reaction can be fast enough without any enzymatic action, unlike conventional methods."
The team expects that the discovery will contribute to further studies of elements and methods for more efficient photosynthesis.
More information: Jae Kyoo Lee et al, Microdroplet fusion mass spectrometry: accelerated kinetics of acid-induced chlorophyll demetallation, Quarterly Reviews of Biophysics (2017). DOI: 10.1017/S0033583517000014
Provided by DGIST (Daegu Gyeongbuk Institute of Science and Technology)